In a very generic way, usability engineering can be described as the user experience and the positive perceptions and reactions that arise from the expected use of a system.
But when we talk about the usability engineering of medical devices we also must have in mind the mitigation of adverse impacts in the users, patients, and environment.
In the medical device context, usability engineering activities goals are focused on understanding the users of the medical devices. They are also focused on the tasks that users need to do in order to use the medical device, the environment where the device is used, the causes and consequences of possible errors while using it, and finally on designing a medical device with a user interface that is as safe as possible.
Why is Usability Engineering becoming so important?
In the past, medical devices used to be much simpler than today.
With the growth in complexity and functionality, the rising of digital interfaces, and the big amount of different medical devices existing, their potential for harm has also grown, as their complexity has increased demand for qualified operators and exact product specification increases.
Nowadays, usability is considered one of the main causes of failure in medical devices, and it is attributed to the Design of medical devices interfaces that do not take into account risks to the users, patients, and their health as well as the environment and economy during the normal and correct use of the device.
The user is the person that interacts in some way with a medical device, sometimes the user is a healthcare professional, but can also be a caregiver or even the patient.
The user interface is anything that the user interacts with in order to use a medical device including displays, instructions for use (IFU), labels, and training materials.
The interaction usually happens physically and visually but can also include sound, vibrations, tactile feeling, and even information interpretations.
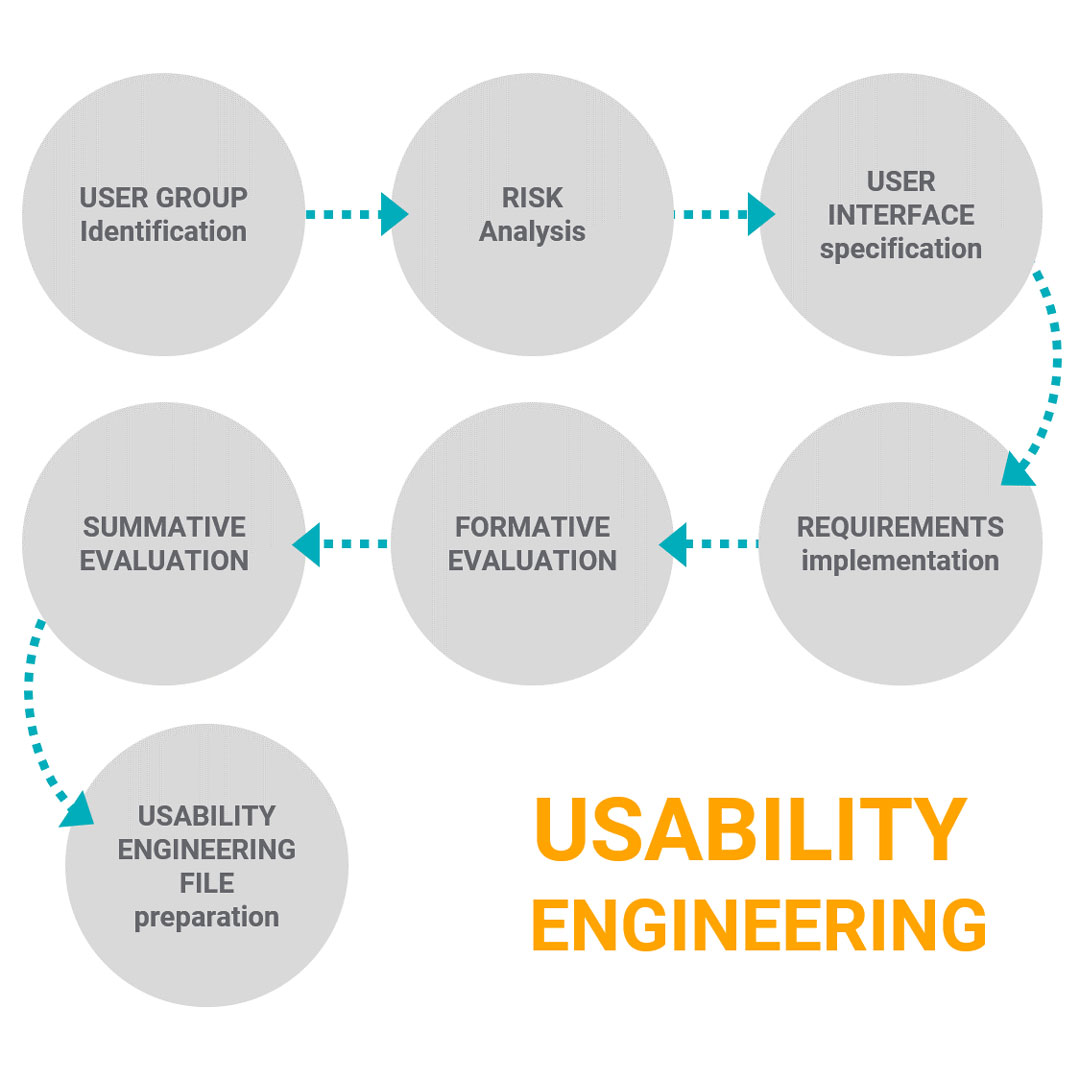
The Usability Engineering process at a glance:
- All usability engineering processes start with the gathering of information about the user groups and anything else that is important for understanding the use of the medical device in one or more specific contexts of use. It is called “Use specification” and can be considered as an extension of the device’s intended use.
- The next step is related to risk analysis and is very similar to the ISO 14971 way to identify and evaluate risks. Actually, usability engineering is part of the medical devices’ risk management activities, so, it is needed to identify the interface characteristics related to safety, potential use errors, foreseeable misuse, hazard situations, and hazard-related-use scenarios. And then select the hazard-related use scenarios to include in the summative evaluation.
According to IEC 62366 use error is a user action while using a medical device that leads to a different result than the intended by the manufacturer or expected by the user. - Proceed with risk control option activities in order to mitigate and reduce as low as possible all risks related to the device interface and use errors. In other words, this step is about determining interface requirements that will be implemented by design activities to reduce the risk related which can be called “User interface specification”.
- Proceed with all requirements “Implementation” during design activities.
- Establish a user interface plan and proceed with the “Formative evaluation”, which as its name suggests is an evaluation made during the interface development. This evaluation provides important feedback that can be used to improve the interface usability, such as new requirements to be implemented into the interface. It can work in a cyclical way between steps 3, 4, and 5 in order to obtain an interface ready for a Summative Evaluation or Usability Validation.
- Finally the “Summative evaluation” demonstrates that all risks associated with mistakes in use due to poor usability interface were reduced as low as possible to an acceptable level. It can be made in a simulated environment with real a product or equivalent by a representative user group with statistical revelation. Summative evaluation aims to obtain Usability validation and remember it is different from Design Validation. Design validation demonstrates that the device meets the defined user needs while Usability Validation demonstrates that the device interface does not lead to an unacceptable risk.
- The last step is to prepare the “Usability Engineering File” that contains all the documentation and records created through all Usability engineering processes.
The Creanova’s device development process integrates Usability Engineering in accordance with IEC 62366 and is able to create and deliver a documentation set that can help you to meet the general and process requirements of this technical standard.
Our deliverables are registered in the main document that keeps traceability of device interface safety-related tasks, use scenarios and their risk analysis, risk evaluation, risk control, and the technical requirements (risk management outputs becoming Design input data). This enables our clients to perform the steps of residual risks, risk-benefit analysis, and overall residual risks acceptance as required per ISO 14971.