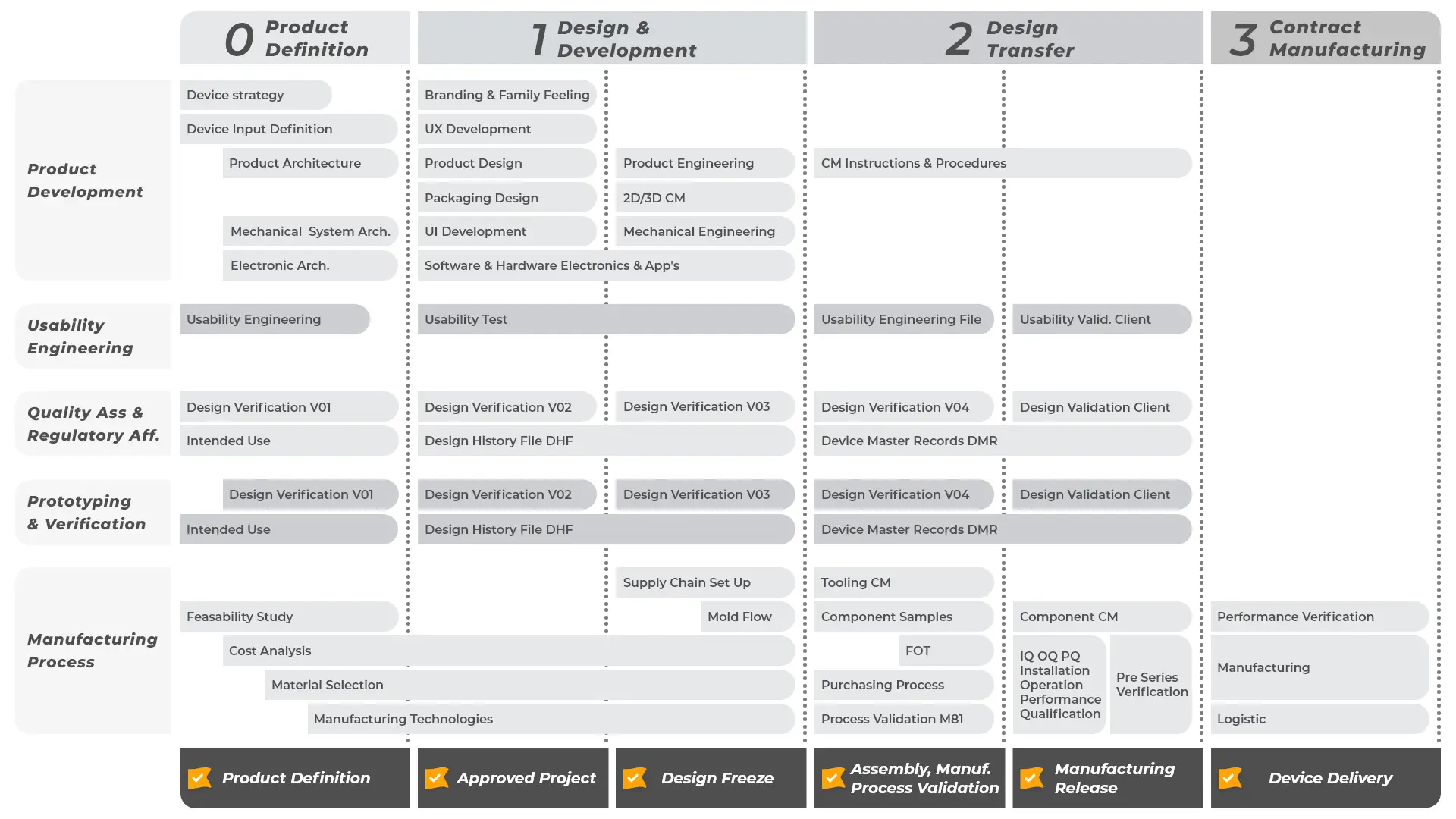
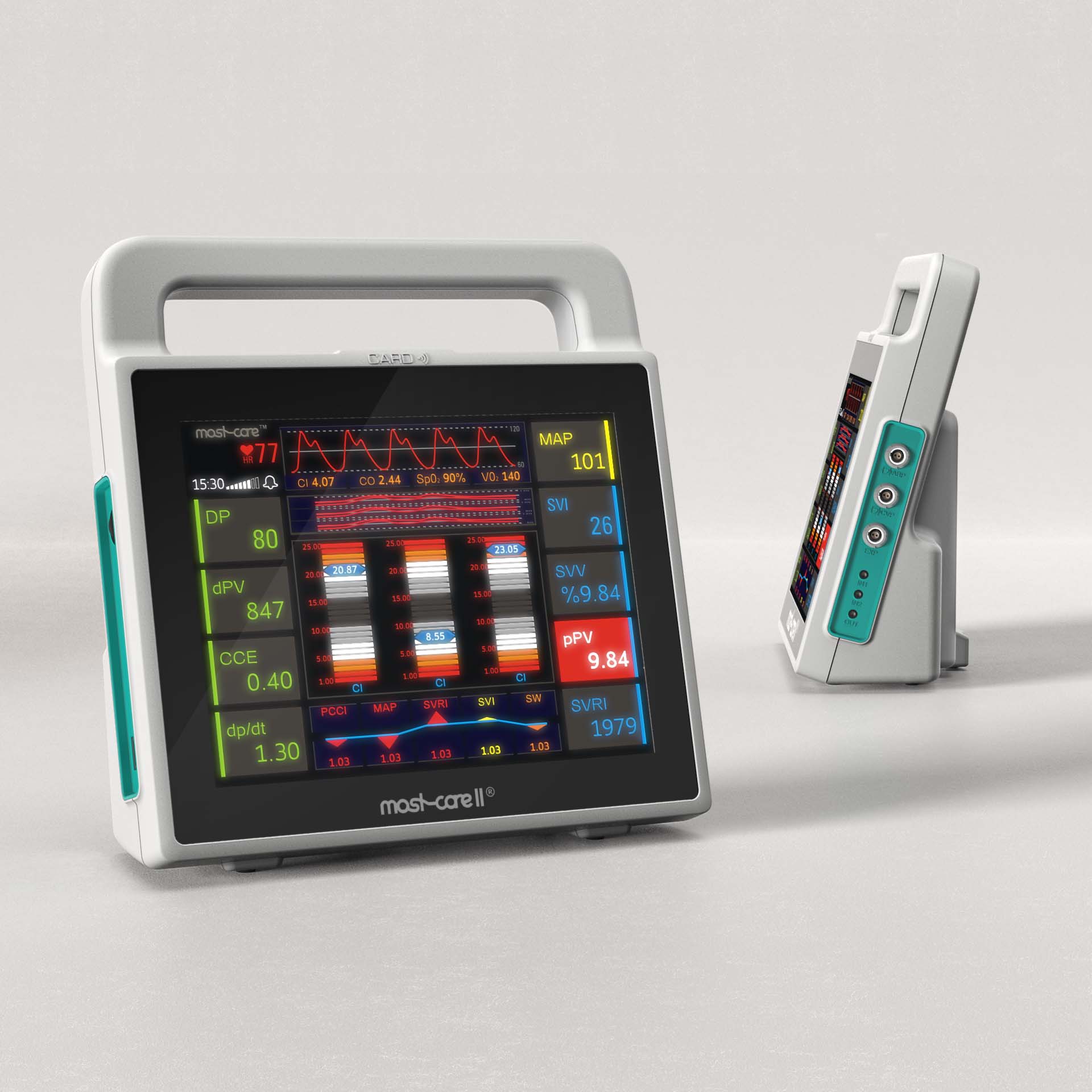
Prototyping is an essential step to ensure the success of your medical device, testing all relevant features, validating the design concept and providing the tangible translation of your idea to present to investors or users. Thanks to our trusted supply chain, Creanova provides multiple prototyping technologies to meet all the possible purposes, from POC (proof of concept) and MVP to aesthetical and functional prototypes up to pre-pilot production. Our skilled engineers support you through the entire prototyping process: from the selection of the most suitable technology to the material identification, going through the quality control, up to assembly and 3D file updating.
Prototypes for validation of:
Proof of Concept
Ergonomics
Aesthetics Functionality (e.g. IP / Drop test)
Prototypes for clinical trials and certification (e.g. FDA, CE)
MVP/ Pre-series production
Case Study
Creanova integrates in its portfolio a full range of services for medical devices, from Industrial Design to Product Development up to Contract Manufacturing.
Our multi-disciplinary team of experts will guide you through our standardized and proven process, always ensuring cost-effectiveness and on-time delivery, in compliance with the complex regulatory environment in Healthcare.
Certified with ISO 9001-ISO 13485
Certified with
ISO 9001 - ISO 13485
Prototyping
Prototyping is an essential step to ensure the success of your medical device, testing all relevant features, validating the design concept and providing the tangible translation of your idea to present to investors or users.
Thanks to our trusted supply chain, Creanova provides multiple prototyping technologies to meet all the possible purposes, from POC (proof of concept) and MVP to aesthetical and functional prototypes up to pre-pilot production.
Our skilled engineers support you through the entire prototyping process: from the selection of the most suitable technology to the material identification, going through the quality control, up to assembly and 3D file updating.
Prototypes for validation of:
Proof of Concept
Ergonomics
Aesthetics
Functionality (e.g. IP / Drop test)
Prototypes for clinical trials and certification (e.g. FDA, CE)
MVP/ Pre-series production