

Gondola Medical Technologies is a Swiss start-up which stands out for its unique non-intrusive technology named AMPS (Automated Mechanical Peripheral Stimulation), treating walking and balance impairment due to neurological diseases, such as Parkinson’s and stroke.
The product consists of two devices, one for each foot, and in two versions, one for B2B market (hospitals, clinics) and one for B2C market (homecare).
Creanova supported Gondola with R&D activities, design and engineering, with a special focus on usability and also took care of the contract manufacturing of the devices at its Italian ISO13485 certified assembly site, completing its full turn-key service from design to contract manufacturing.

The design and layout analysis phase required R&D activities to develop the innovative medical solution and respond to the following challenges:
Our designers and engineers’ team implemented new functionalities to allow the easy adjustment of the medical device for each patient and reach the best accuracy.
On the other hand, the best usability was achieved through the analysis of:
Regarding the engineering of the B2B healthcare device, the user journey was shaped to make it as easy-to-use as possible and leveraging icons and colour to make the operations intuitive and the usability effective.
On patient side, great attention was given to the comfort and softness of the footprint. The medical device has been lightened and a base was added to make it self-supporting during the therapy and finally relieve the patient.
On the other side, the Homecare device is characterized by clean and compact design and the procedure is extremely simplified so that the patient just closes the band and presses the Start button.
The layout and design phase was validated through a POC (proof o concept) to practically assess the ergonomics, volume mechanism and functioning.
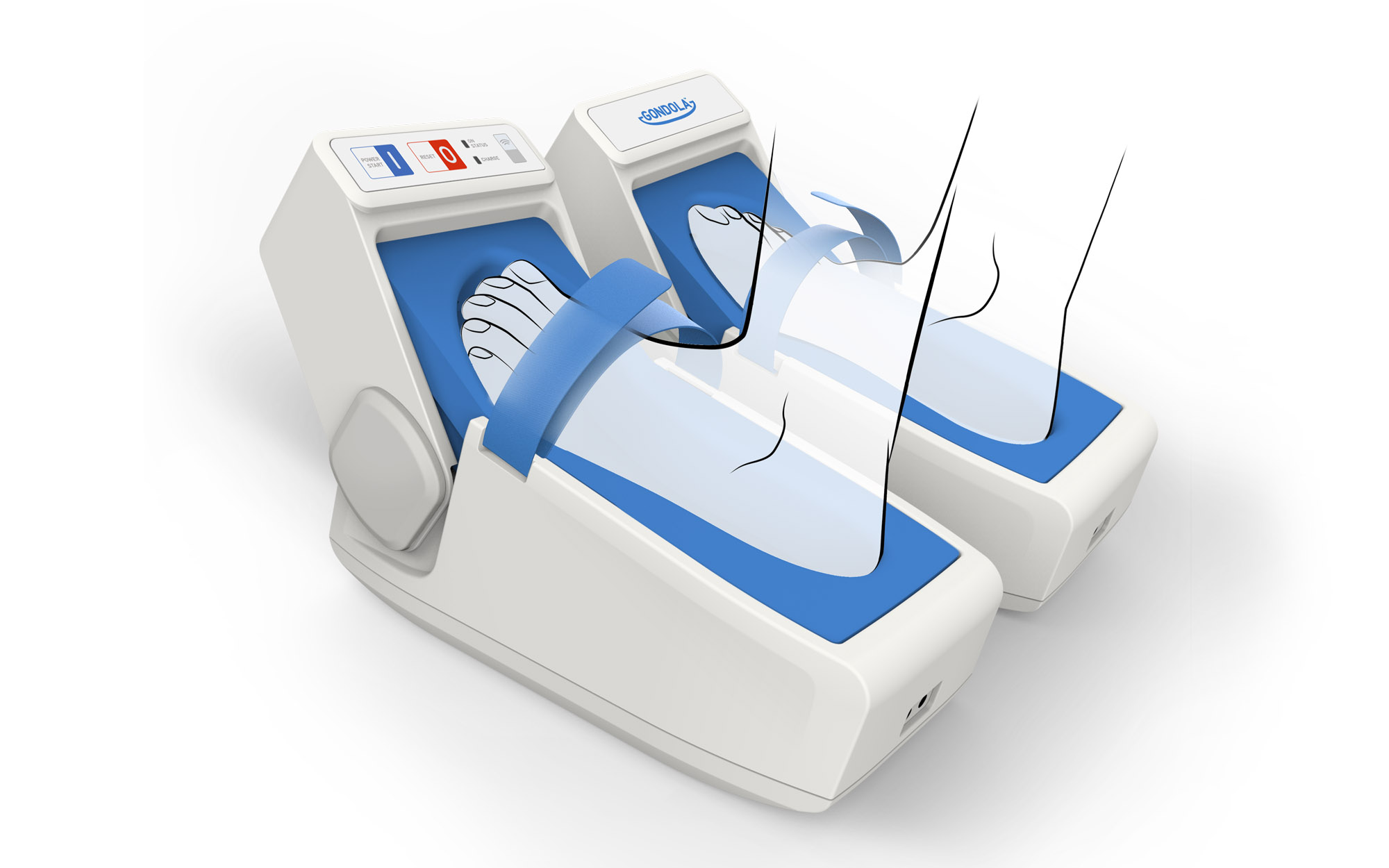
The engineering phase of the wearable medical device revolved around four main goals:
Our engineering team has made possible the easy device adjustment, without the need of any tool. For cost optimization, the two elements of each device were designed using the same shells. In addition, the silicone injection molding were chosen as the production technology to reduce initial investment.
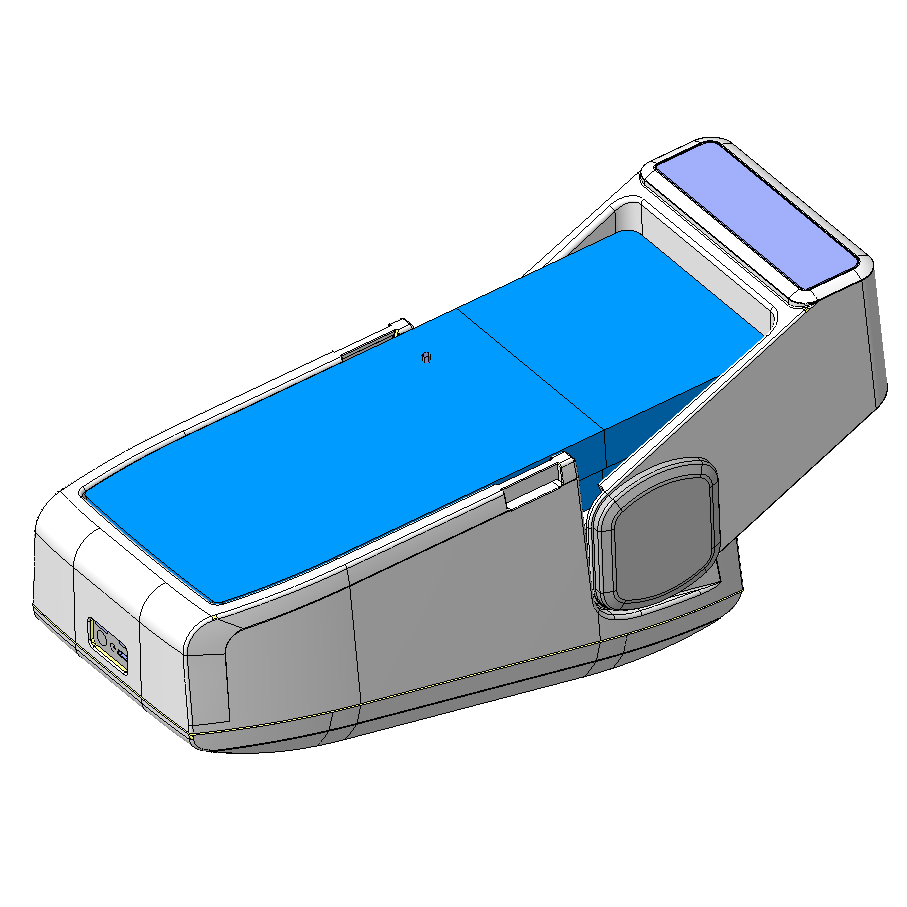
Functional prototypes were used to verify with the Client the usability and functionality and to submit the medical devices to the Notify Body.
These validation prototypes were subjected to several tests, aimed to assigning the medical certification to the device:

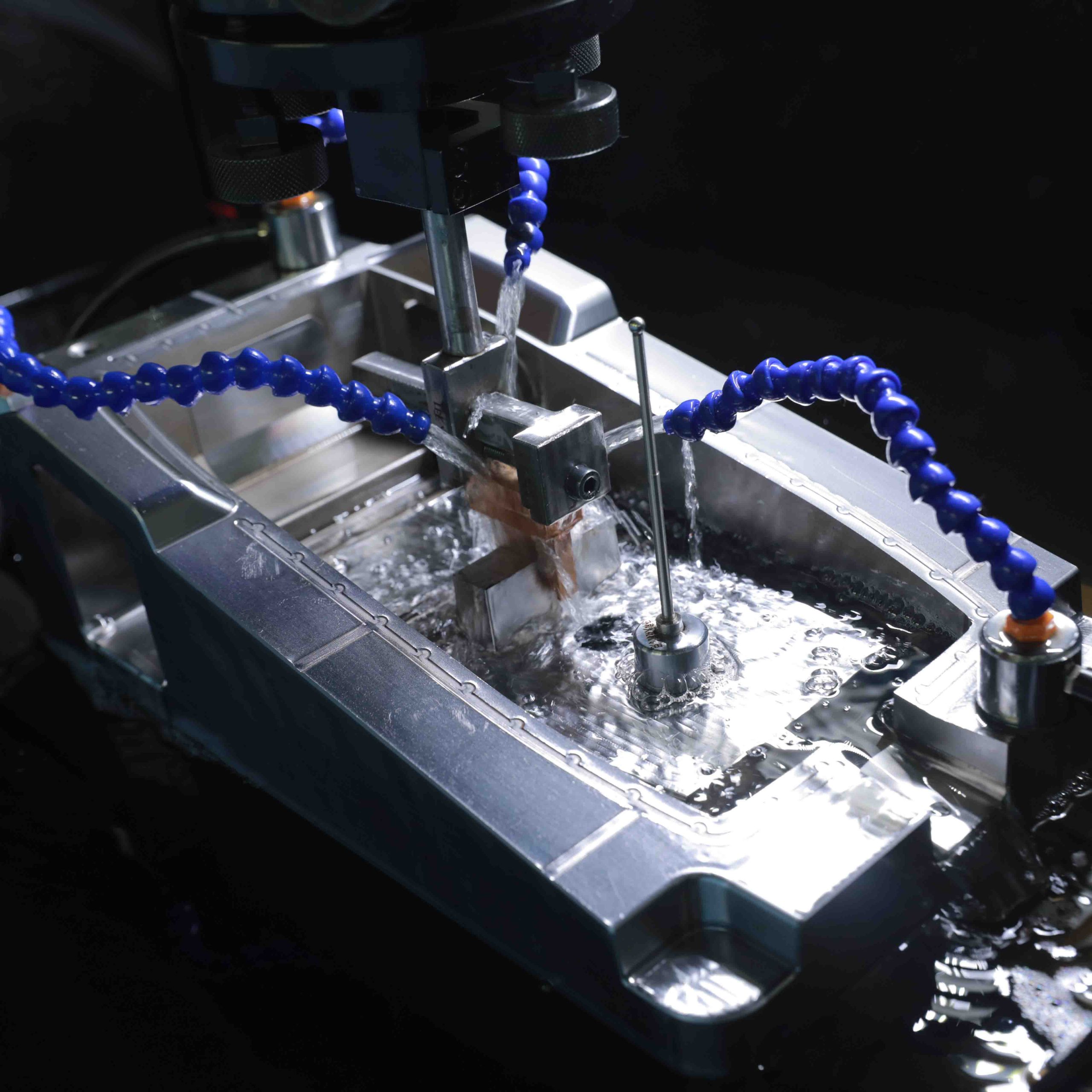
Our production specialists managed the tooling, production and assembly of this innovative wearable device, providing the following services:

tel. +39 0315000293
info@creanova.com
Creanova S.r.l.
Via Antonio Magni 54
22100 Como, Italy
VAT. IT 03103770131
SDI: USAL8PV
Legal Aspect and Privacy Policy Creanova Copyright © 2021

tel. +39 0315000293
info@creanova.com
Creanova S.r.l.
Via Antonio Magni 54 – 22100 Como, Italy
VAT. IT 03103770131
SDI: SUBM70N
Creanova ISO 9001 | Creaproduct ISO 9001, ISO 13485 | Creanova d.o.o. ISO 13485, ISO 14644 | Legal Aspect and Privacy Policy Creanova Copyright © 2021

tel. +39 0315000293
info@creanova.com
tel. +39 0315000293
info@creanova.com
Creanova ISO 9001 | Creaproduct ISO 9001, ISO 13485 | Creanova d.o.o. ISO 13485, ISO 14644 | Legal Aspect and Privacy Policy Creanova Copyright © 2021