The 360° medical device development process is completed by our manufacturing and assembly services for pre-production up to large scale production, empowered by Creanova’s fully owned assembly facilities in Italy and Serbia (certified with ISO 13485). Creanova d.o.o., thanks to its location in Serbia, ensures competitive costs, high quality and short delivery times, and guides our clients until the shipment of the packaged end-product.
Parts production
PCB production (Surface-Mount Technology) incl. firmware programming
Standard components purchasing
Quality Control (Incoming, In-line and Final)
Assembly
Cleanroom production & assembly (ISO 14644)
Testing and validation of assembled device
Product traceability (ISO 13485)
Labelling & packaging
Storage & Logistics
Case Study
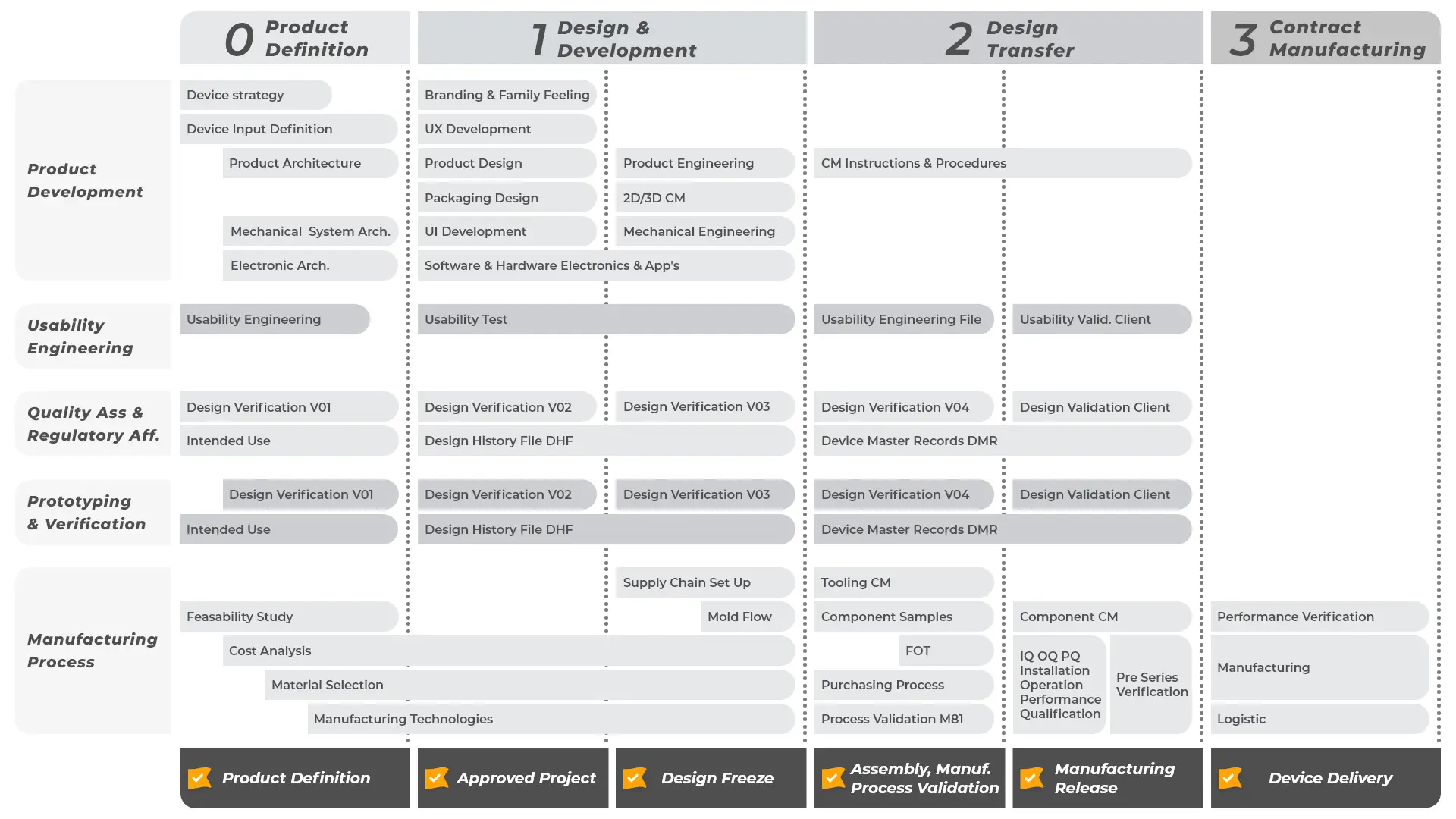
Creanova integrates in its portfolio a full range of services for medical devices, from Industrial Design to Product Development up to Contract Manufacturing.
Our multi-disciplinary team of experts will guide you through our standardized and proven process, always ensuring cost-effectiveness and on-time delivery, in compliance with the complex regulatory environment in Healthcare.
Certified with ISO 9001-ISO 13485
Certified with
ISO 9001 - ISO 13485
Production & Assembly
The 360° medical device development process is completed by our manufacturing and assembly services for pre-production up to large scale production, empowered by Creanova’s fully owned assembly facilities in Italy and Serbia (certified with ISO 13485).
Creanova d.o.o., thanks to its location in Serbia, ensures competitive costs, high quality and short delivery times, and guides our clients until the shipment of the packaged end-product.
Parts production
PCB production (Surface-Mount Technology) incl. firmware programming
Standard components purchasing
Quality Control (Incoming, In-line and Final)
Assembly
Cleanroom production & assembly (ISO 14644)
Testing and validation of assembled device
Product traceability (ISO 13485)
Labelling & packaging
Storage & Logistics