
Drop testing for your medical device development
Medical Electrical Equipment must follow generic standards and regulations to obtain specific certifications that allow it to be marketed (e.g. CE marking for Europe). In

Medical Electrical Equipment must follow generic standards and regulations to obtain specific certifications that allow it to be marketed (e.g. CE marking for Europe). In

The Global Dental Equipment Market is expected to register a CAGR of ~3.3% during the period 2020 to 2028 and will reach $8 Bn in
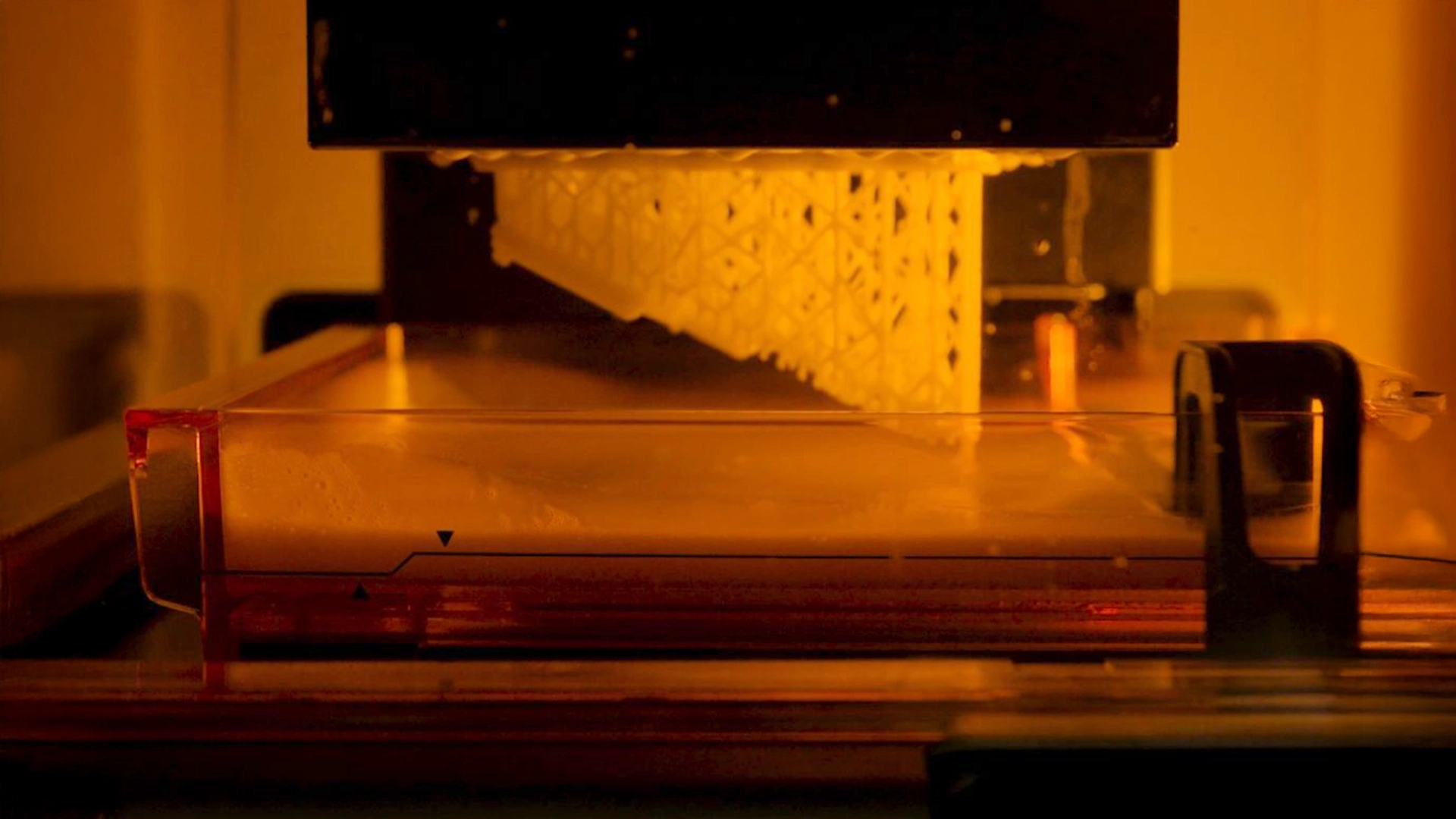
Medical Device Rapid prototyping is a method used by companies to turn simple ideas into realistic POC (proof of concept). It enables Medical device design

Color for a medical device is often overlooked when designing and developing a device of that type, but it can play an important role in
Many Med-Tech companies contact the medical contract manufacturer to find out the cost of making their medical device without giving any further information. This is

The advances that have occurred in digital, biomedical and data technologies bode well for the medical device development process. It must be said, however, that
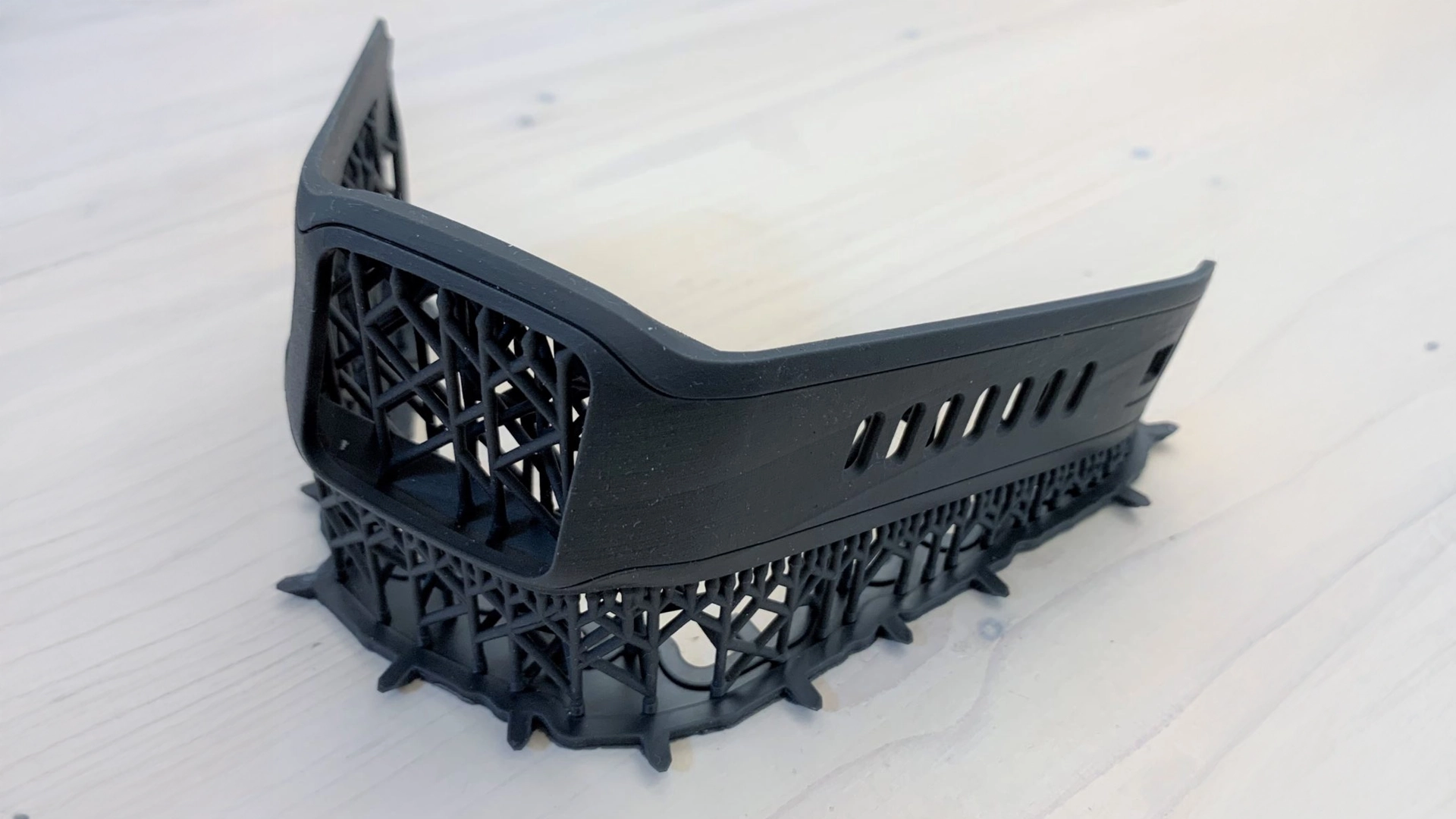
Medical device prototyping is useful for testing the design and performance of a product beforehand. With prototyping, you can test materials and assess the potential market

Prototypes provide proof-of-concept, certifications, tests for engineered model, design patterns and benchmarks so they are very important in the medical device design, engineering and manufacturing
The life sciences are concerned with the study of living organisms and their life processes. The life sciences industry includes companies, businesses and research institutions

Med-Tech companies invest time and money in producing a medical device that is attractive to consumers or medical professionals—it usually needs to be ergonomic, eye-catching,