
Medical Device Design Strategy: From Insights to Market
Why Medical Device Design Strategy Matters A medical device design strategy is far more than a creative exercise. It is a structured framework that guides

Why Medical Device Design Strategy Matters A medical device design strategy is far more than a creative exercise. It is a structured framework that guides

In medical device development, choosing how to structure your supplier ecosystem is not a secondary operational detail. It is a strategic decision that affects time

The design challenge in the medical sector lies in combining safety and functionality with innovation. Creanova approaches design by focusing on the user experience. We

In the medical device sector, regulatory documentation is the gateway to market access and ultimately to patients. Yet, preparing and maintaining submission dossiers, clinical evaluation

In our previous article, Simplifying Medical Device Design: Reducing Complexity for Safety and Efficiency, we explored the theory behind medical device complexity and how simplification
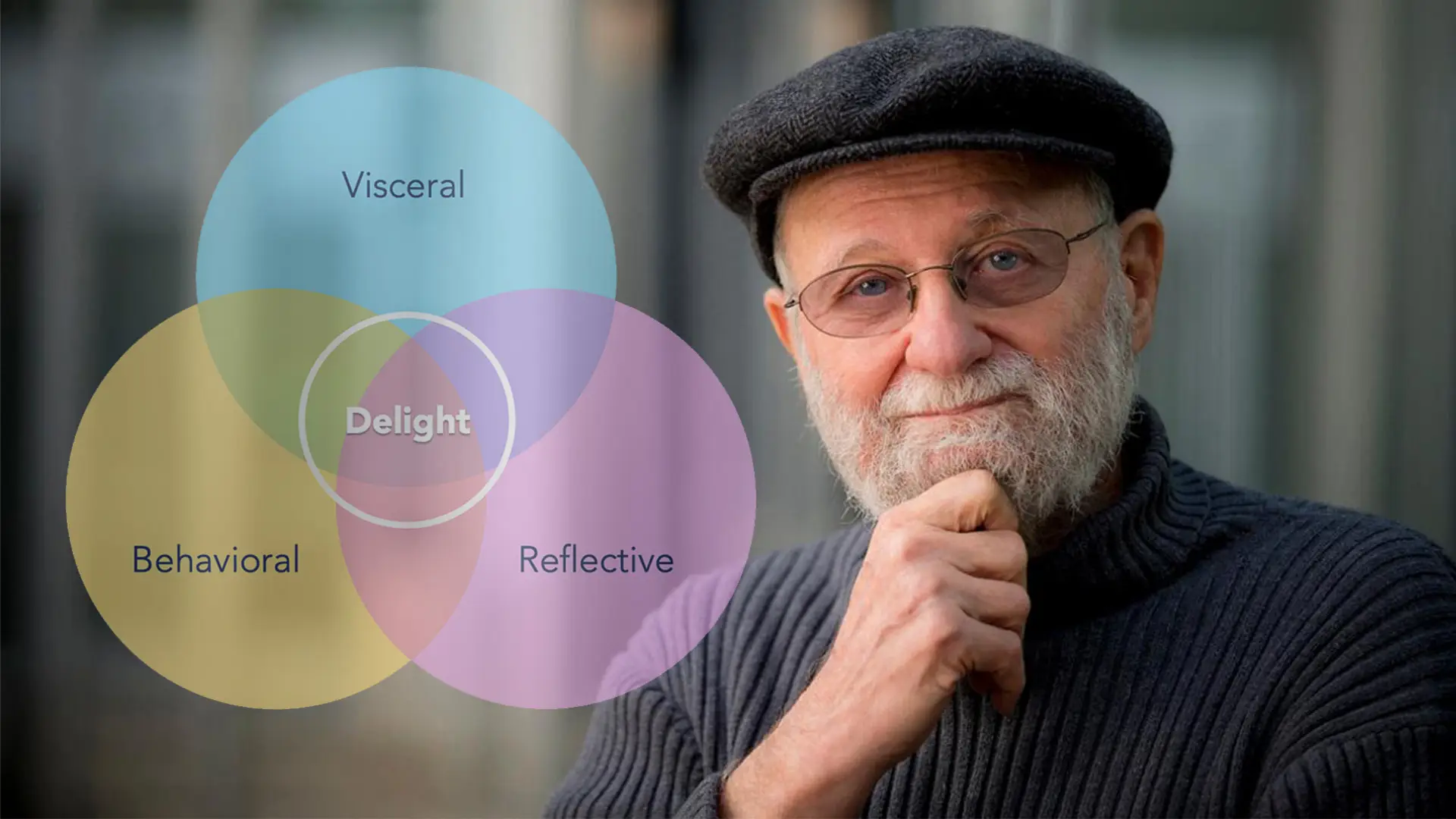
When we think of medical device design, we often imagine safety standards, risk management, and strict regulations. Yet, Donald Norman’s concept of emotional design reminds

Embark on a visionary journey into the cutting edge of the aesthetic trends in medical device design, where innovation is driven by the conversion of

Soft materials play an important role in medical device development, especially in applications that require flexibility, skin contact, and patient safety. Including other various applications,
Iterative Design and AI in Medical Device Development Design is never a destination—it’s a continuous journey of prototyping, testing, and improving.In medical device development, this

In the medical sector, the role of concept design plays a fundamental role in defining the identity and functionality of the product. Although the role