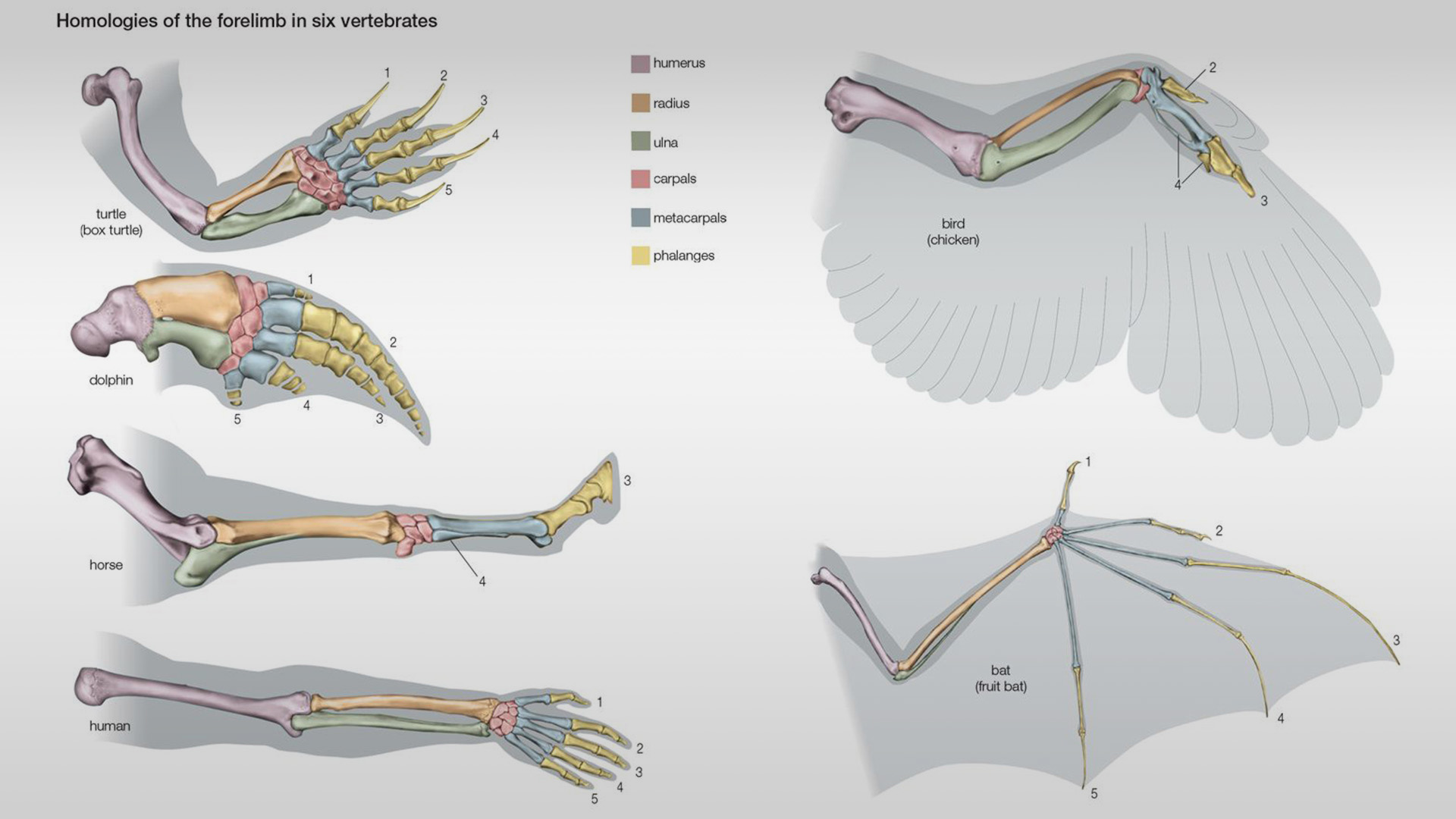
Iterative Design and AI in Medical Device Development
Design is never a destination—it’s a continuous journey of prototyping, testing, and improving.
In medical device development, this approach is crucial: safety, usability, and performance depend on our ability to iterate quickly, validate rigorously, and learn constantly.
Today, artificial intelligence (AI) in medical devices embraces the same principle: train, test, refine, repeat. In this article, we explore how iterative design methods and AI cycles reinforce each other to accelerate healthcare innovation.

Why Iterative Design Matters in Medical Devices and AI
Iteration is the beating heart of both medical product design and AI model training.
Definition: Iteration means creating successive versions with the goal of learning.
Benefits in medical device development:
- Continuous feedback uncovers usability risks early.
- Early fixes reduce cost and time-to-market.
- Evidence-based decisions strengthen stakeholder confidence.
- Evolutionary mindset: like natural selection, each design cycle keeps what works and discards what doesn’t.
👉 In medtech, iteration supports regulatory compliance, patient safety, and design excellence.

“We are all prototypes”: humans, design and AI models
The prototype mindset applies not only to products, but also to people and AI models.
- Growth mindset: designers, engineers, clinicians, and AI algorithms improve with each test and feedback loop.
- Cross-functional learning: when human teams and AI tools collaborate, bias decreases and insights multiply.
- Example: a wearable diagnostic device may begin with multiple interface prototypes. Usability tests narrow down the options, while AI simulations predict clinical scenarios and inform the next iteration.

Agile Methods for Iterative Medical Device Design and AI Training
How can iteration become daily practice?
- Agile sprints: short cycles with testable artifacts and structured reviews.
- Prototype ladder: from sketches → CAD models → functional prototypes. Use the simplest version that answers the design question.
- Feedback loops: Design → Test → Analyze → Improve. Document issues, decisions, and metrics to prevent regressions.
- AI cycles: model training, prompt iteration, and performance tuning mirror the iterative workflow of medical device design.
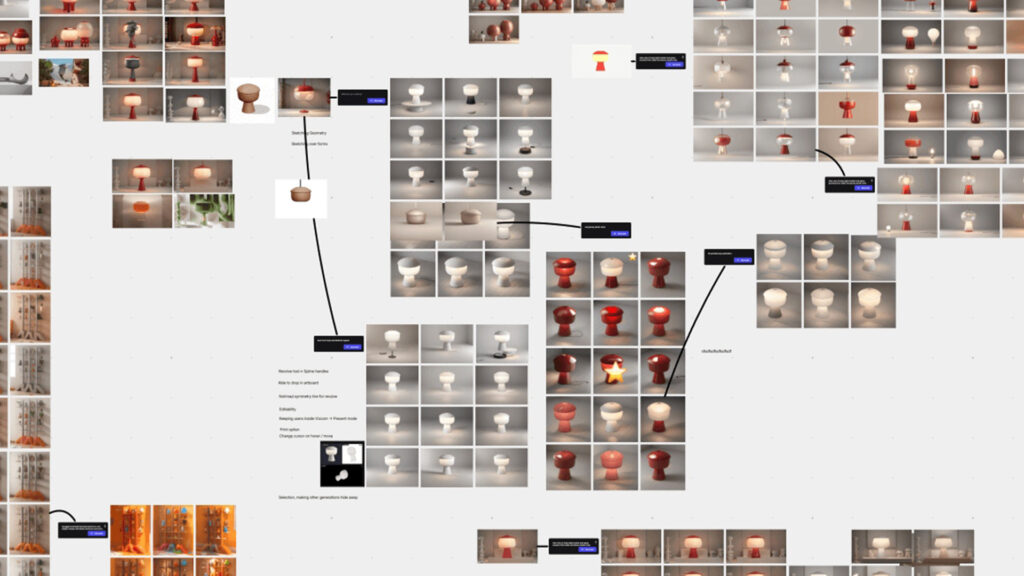
Strategic benefits for Creanova, clients, and AI adoption
By combining iterative medical device design and AI-driven innovation, companies gain:
- Quality & safety: multiple cycles lead to robust, validated devices (aligned with ISO 13485 and FDA design control).
- Efficiency: AI accelerates data analysis, usability evaluation, and risk detection.
- Innovation: teams test bold ideas quickly; AI generates new variants; experts select the most promising.
- Traceability: every iteration strengthens compliance and Design History Files (DHF).
Conclusion: Turning Iteration into Innovation
Projects, like people and AI models, are never truly finished—they evolve. Iterative design in medtech transforms ideas into safe, compliant, and market-ready products.
👉 Adopt the prototype mindset with Creanova—your partner in iterative medical device design and AI-driven healthcare innovation.