At Creanova, we recognize that while innovation often fuels progress in healthcare technology, unchecked complexity can become a silent obstacle in product development. In the field of medical device design, excessive complexity leads to higher manufacturing costs, usability issues, maintenance challenges, and regulatory delays.
That’s where simplified medical device design comes into play.
By reducing unnecessary intricacy, manufacturers can improve device reliability, speed up time-to-market, and reduce the risk of human error. When applied strategically, simplification in medical device design doesn’t mean doing less—it means designing smarter to enhance usability, safety, efficiency, and long-term maintainability.
In this article, we explore what design complexity really means in a medical context, why it matters, and how simplified medical device design delivers better outcomes for manufacturers, healthcare providers, and ultimately, patients.
What Is Device Complexity in Medical Device Design?
Device complexity refers to the number and interaction of components, systems, software layers, and interface elements within a medical product. In the context of medical device design simplification, complexity is not inherently negative—but unmanaged complexity can compromise performance, safety, and scalability across the medical product lifecycle.
A complex medical device might include:
- A high number of mechanical or electronic components
- Redundant or overlapping subsystems
- Non-intuitive or inconsistent user interfaces
- Sophisticated firmware with tightly coupled logic
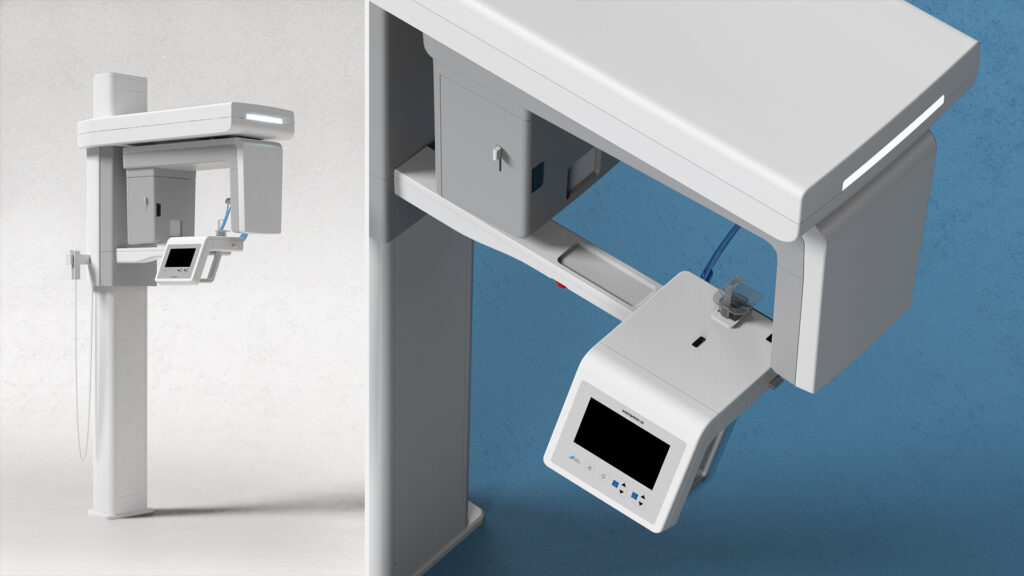
Each of these features may serve specific technical needs. However, without careful engineering and streamlined design, they introduce friction and inefficiencies across the device’s entire lifecycle:
- Design & Development: Increased engineering workload, slower time-to-market, and added challenges in design for manufacturing (DFM)
- Manufacturing: Higher production costs, multi-step assembly, and elevated risk of assembly errors or defects
- Usability: More training needed for healthcare professionals and end users, and a higher likelihood of human error due to interface complexity
- Maintenance & Lifecycle: Difficult field servicing, increased failure points, and a reduced product lifespan
- Regulatory Compliance: More extensive technical documentation and longer review cycles for regulatory compliance in medical devices
In short, medical product design simplification is not about removing features—it’s about reducing unnecessary intricacies. By implementing modularity in medical devices, optimizing system integration, and adopting intuitive device interfaces, designers can enhance the usability of medical devices, increase their safety, and simplify ongoing maintenance and regulatory tasks.
Why Simplifying Medical Devices Is Strategic
Simplicity in medical device design is not merely aesthetic—it is functional strategy.
A simplified device:
- Is easier and faster to produce
- Requires fewer resources to assemble and maintain
- Offers a more intuitive user experience, reducing training needs and human error
- Enables easier compliance with medical standards and regulatory frameworks
- Is more scalable, especially for multi-version or modular development
As the Italian designer Bruno Munari said, “Complicating is easy, simplifying is difficult.”

This is especially true in medical device design, where complexity can become a liability, impacting safety, usability, and commercial viability. At Creanova, we view simplification as an opportunity to combine design thinking, engineering best practices, and user-centered strategies into a single, cohesive product development vision.
At Creanova, we view simplification as an opportunity to combine design thinking, engineering best practices, and user-centered strategies into a single, cohesive product development vision.
5 Proven Methods to Reduce Medical Device Complexity
There are several proven methods for reducing complexity while preserving (or enhancing) performance:
1. Component Integration
Reducing the number of individual parts in a device streamlines manufacturing, reduces assembly errors, and improves long-term reliability.
For instance, using System-on-Chip (SoC) technology allows multiple digital functions (e.g., data processing, wireless communication, power management) to be embedded into a single chip. This eliminates the need for multiple discrete modules, lowers the failure rate, and decreases device footprint.
2. User-Centered Interface Design
User interaction is one of the most common sources of medical error. A poorly designed interface with ambiguous icons, inconsistent feedback, or complex menu navigation can confuse users—especially in high-pressure medical environments.
A simplified interface:
- Presents only the essential information
- Uses intuitive icons and clear labeling
- Follows logical workflows
- Offers immediate visual and auditory feedback
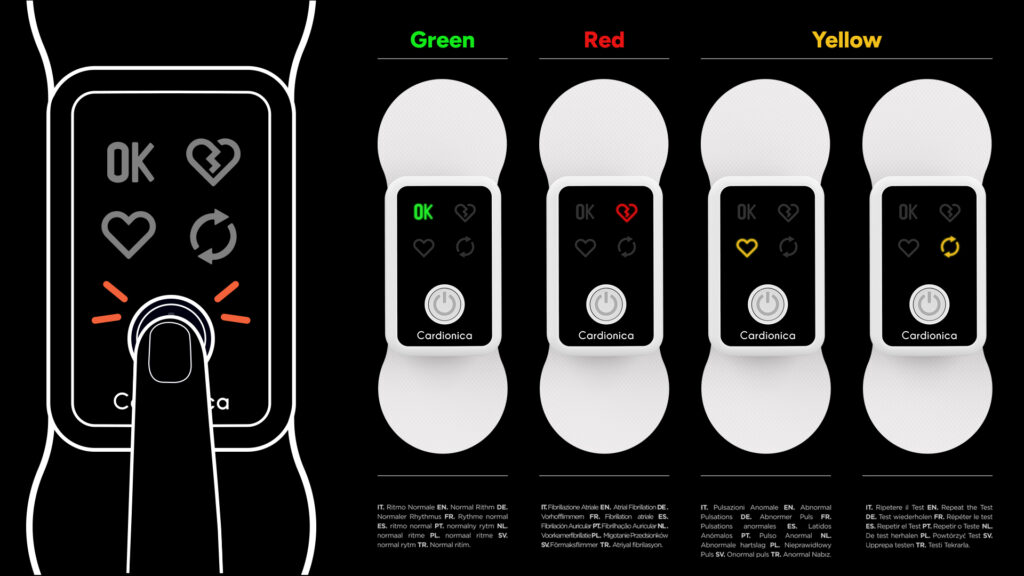
The result is a device that instills user confidence, reduces training time, and enhances patient outcomes.
3. Software and Firmware Optimization
Modern medical devices rely heavily on embedded software. However, software bloat can lead to:
- Slower performance
- Harder debugging and validation
- Increased cybersecurity vulnerabilities
At Creanova, we follow a modular software architecture that separates core functions into discrete, updatable modules. This allows for:
- Easier upgrades and patches
- Simplified regulatory validation
- Lower maintenance effort over time
4. Streamlined Manufacturing Processes
Manufacturing efficiency begins at the design stage. Devices that are designed for assembly (DFA) and manufacturing (DFM) can be produced faster and with greater repeatability.
We focus on:
- Using standardized components
- Designing parts that fit together intuitively
- Supporting automated or semi-automated assembly
- Minimizing the need for manual calibration or configuration
This strategy reduces lead times, lowers cost, and improves supply chain resilience.
5. Simplified Regulatory Compliance
Complex designs can trigger complex regulatory reviews. For example, a device with multiple interchangeable modules or software-dependent features may require extensive documentation, clinical testing, or validation.
Simplified devices—built with proven components and clear functionality—often move faster through FDA, CE, or MDR approval processes.
By focusing on simplicity, Creanova helps clients avoid regulatory pitfalls, accelerating time-to-market and reducing compliance costs.
The Role of Battery Selection in Portable Medical Devices
Battery selection plays a central role in the complexity and usability of portable medical devices.
The battery is the power source that enables devices to function in non-clinical environments, making its performance and lifespan crucial for reliable device operation.
However, selecting the right battery involves navigating a range of technical challenges – balancing power requirements, device size, weight, and safety concerns.
Key considerations include:
- Energy Density: To ensure adequate runtime in compact form factors
- Safety: Avoiding overheating, overcharging, or leakage
- Size and Weight: Maintaining portability without sacrificing performance
- Integration: Fitting the battery into the internal architecture without complex wiring or additional protection systems
- Replaceability or Rechargeability: Depending on the intended use case
- Creanova applies a systematic, use-case-based approach to battery selection. We evaluate:
- Power consumption profiles
- Operating environments (clinical, home use, mobile)
- Thermal management needs
- Safety certifications and compliance
The right battery choice enables greater autonomy, easier maintenance, and a cleaner internal architecture, further supporting simplification.

Benefits of Simplified Medical Device Design
In conclusion, we can summarize the main advantages of the simplification process as follows:
- Cost Efficiency: Fewer components and simpler assembly reduce production costs.
- Improved Reliability: Integrated systems reduce the risk of component failure.
- User Experience: Intuitive operation minimizes training and error.
- Time-to-Market: Streamlined validation and production accelerate launches.
- Regulatory Compliance: Easier documentation and certification.
- Hygiene and Maintenance: Cleaner surfaces and fewer crevices simplify cleaning.
Designing with Purpose, Not Complexity
At Creanova, we don’t view simplification as a constraint—it’s a strategic advantage. Simplified medical device design is not about reducing innovation, but about making innovation more usable, manufacturable, and safe.
When simplification is intentional, the results are clear: reduced human error, accelerated time-to-market, lower production costs, and intuitive tools that empower healthcare professionals and patients.
In the next article, we’ll present a real-world case study on simplifying the design of a portable monitoring device – showcasing how Creanova’s principles translate into concrete results, from battery integration to user interface and hygiene-focused design.
Stay tuned for Part 2!

