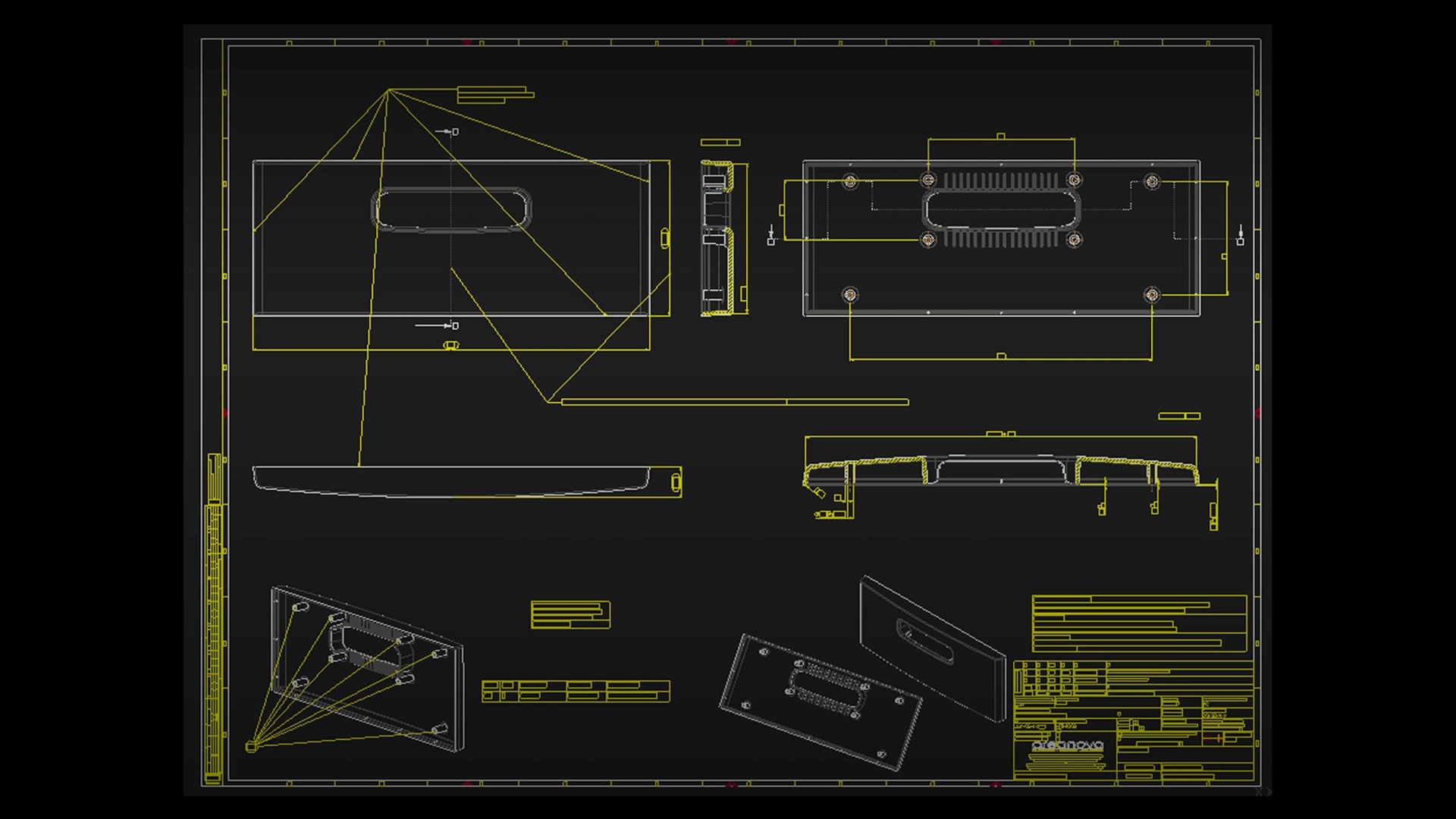
For a long time, 2D drawings were the only way to convey product design information. In the last twenty years, 3D models have been a game changer. So how can 2D be used in the medical device development process in 2021?
2D drawings simplify the new 3D models and directly present key information, such as dimensions and tolerances, useful for important decisions. 2D medical devices drawings have different graphics than medical device 3D models and also contain manufacturing instructions. The 2D views also have all the references and detailed dimensions so you don’t always have to rely on a 3D model. When manufacturers want to know the quality of a part, or whether all dimensions have been respected, they need the 2D drawings. Also, on many occasions (e.g. in a shop floor) hard copies are preferred, so cheap and easy-to-print 2D medical devices drawings are still very useful.
Here are 4 reasons why you still need 2D drawings in medical device engineering:
- Flexible Design
One of the advantages of using 2D CAD is that the medical device design can be changed easily. Unlike traditional, paper-based or sophisticated 3D medical devices CAD models, on a digital platform the layout can be changed in a few clicks. This is mainly useful at the beginning of the medical device development process. You can experiment with different options until you are completely satisfied with the design, addressing potential product issues and
assuming how the elements would fit together in the final product, before finalising the
development in 3D CAD. - Easy to Use
3D CAD tools offer numerous functions for medical device engineering, but these are not needed in the early stages of medical device development. 2D CAD tools, on the other hand, are important at the beginning as they contain essential functions that are easy to understand and use. They include a layer, line types, line weights and more.
Thanks to the 2D method, non-expert engineers/CAD users can draw some ideas to start communicating with other stakeholders and technical staff for further development. - Error-Free
As there are few manual calculations, 2D CAD does not permit many errors. Medical device engineers can digitally create various (mechanical) components very precisely and then modify them according to requirements without making mistakes.
As a result, designs are more accurate and faster and have minimal manual input. - Time and Cost-Saving – 2D CAD software allows medical device engineering to focus on critical areas. In addition, digital drawings can be shared easily between the teams involved. This means that everyone is updated in the same way and if someone finds errors, they can be dealt with quickly and relatively easily. Thus, the whole process becomes much faster.
Utilizing both 2D and 3D can help achieve medical project optimization as it improves interoperability and reduces manual updates. 2D medical devices CAD can help at different stages of medical device development: in the early stage it allows you to quickly draw concepts and assess feasibility; in the transfer-to-production stage it helps to create drawings for shop floor requirements and further share them with team members. In return, 3D medical devices CAD
tools can help with designing testing (e.g. FMEA for structural calculation), visualization (e.g. photorealistic renderings), error detection (e.g. automatic interference checking) and manufacturability optimization (e.g. mold flow analysis). Thus, although 3D CAD models are widespread, 2D drawings are still necessary for medical device development. Creanova has a team of engineers and manufacturing experts happy to help you with all your
design and engineering needs.
For 20 years, Creanova has been supporting Med-Tech companies throughout the entire medical device development process, from the early stages of feasibility studies to industrial design, medical device engineering (2D and 3D CAD) and prototyping, up to tooling and contract manufacturing (ISO 13485 certified).
So get in touch with us and let’s work together to make your project a reality!