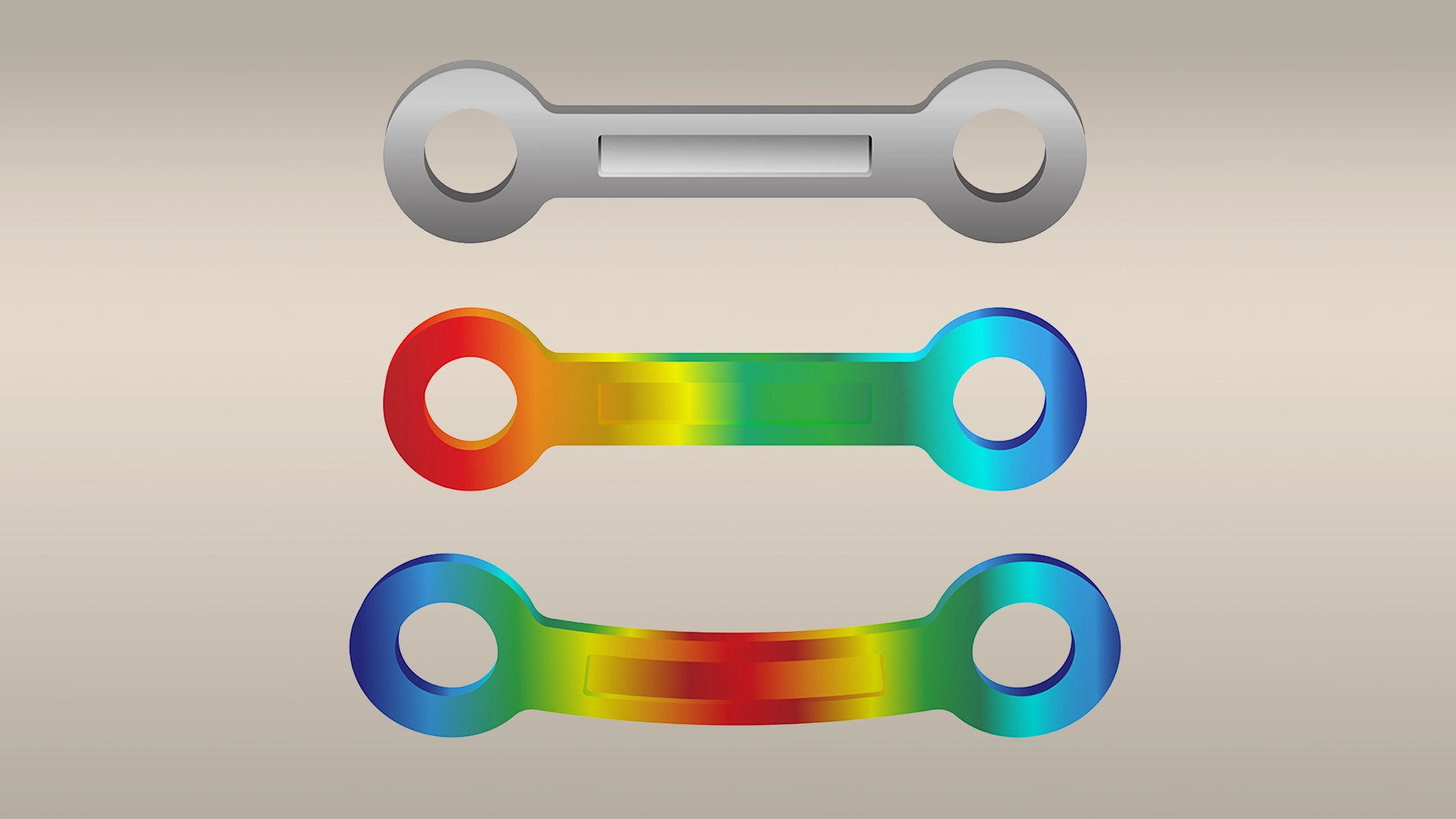
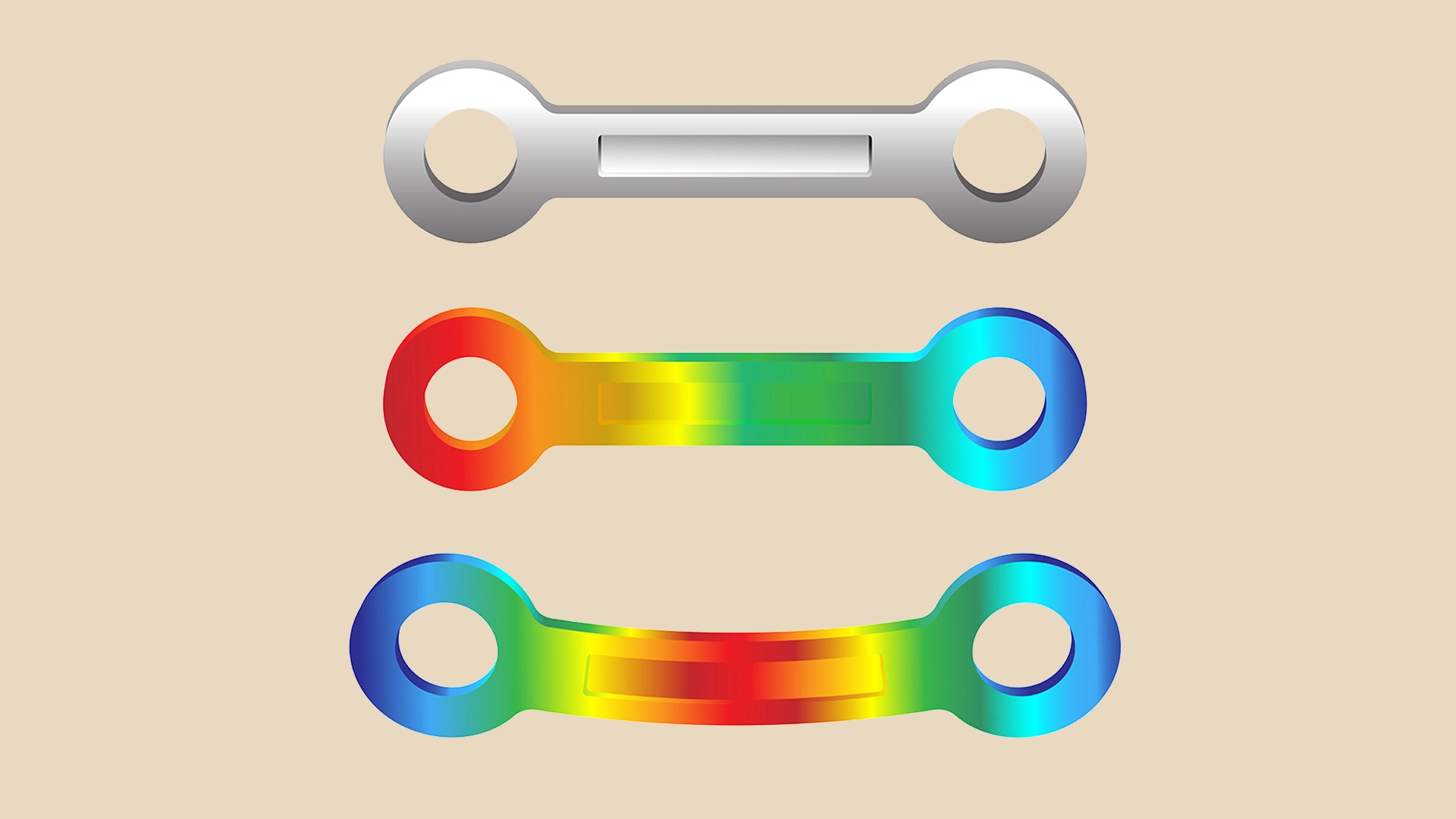
FEA stands for Finite Element Analysis and is a tool commonly used by engineers to simulate and evaluate the key properties and functions of products, such as fluid flow, vibrations, heat conduction, and electrostatics, and also helps in the medical device development. The task of FEA is to simplify a complex part by splitting it into a finite number of small simple bricks called elements. The elements are then analysed individually using numerical computerised methods. During the medical device finite element analysis, the software tries to understand how the product reacts to conditions such as leads, displacements and heat by analysing each element and its neighbouring elements until an overall view is obtained. Once the software has drawn its conclusions, it returns results such as stresses, energies and deformations of the innovative medical device. When the part is stressed, the medical device FEA allows the designer to see its weaknesses. Once these results are obtained, the design can be modified, for example by removing material where it is not needed and putting it where it is needed.
FEA can be used in medical device engineering using animations and coloured contour graphics.
HOW DOES IT WORK?
The Medical device FEA procedure usually consists of three steps:
- In the pre-processing phase, information such as geometry, mesh element type, material properties, boundary conditions and applied load must be entered. In addition, stand-alone FEA software, or those bundled with CAD (computer aided design) software packages, import 3D CAD solid models for pre-processing and analysis.
- In the analysis phase, matrix equations are generated to describe the behaviour of each finite element, then all the equations of the various elements are assembled to give an overview of the structure, and finally the equations are solved.
- In the final post-processing phase, the solution is processed, along with the graphical interpretation of the variable on the CAD model in modern FEA software packages.
WHY IS FEA SO IMPORTANT IN THE INNOVATIVE MEDICAL DEVICES DEVELOPMENT?
FEA, which is also a virtual testing platform, can be used to speed up the design process. Before making any prototypes, FEA can be used to model the structural behaviour of complex systems and geometries and lower the medical device development risks of product features and functions. It reduces the risk that arises from the desire to implement adventurous ideas and allows potential problems to be detected early in the process so that design changes can be made while they are still inexpensive. In later stages, the medical device FEA becomes a powerful optimisation tool along with prototyping and testing. It can also be used to test worst case scenarios for which you have no physical evidence.
Here are the main benefits of Medical device Finite Element Analysis:
1. Designing Parts Exactly for Their Function
An FEA reveals the strengths and weaknesses of a part by testing its reaction to various forces. With the results from this analysis, a minimum viable part can be created, i.e. a part that uses exactly the right amount of material and functions exactly as it should.
2. Save Time and Money on Your Overall Medical Device Development Process
By designing parts exactly to fulfill their purpose, you use less material and save time and money on production. Finite Element Analysis allows designers and engineers to refine a design, prior to prototyping or manufacture, creating innovation in medical devices.
Today many medical devices are made of injection moulded plastics and changes are expensive and time-consuming. Therefore, avoiding injection mould modifications by using FEA also saves a lot of time.
3. Supporting Your medical device Submission
Medical devices must undergo rigorous testing to prove that they are safe and effective.
As mentioned before, a medical device FEA can reveal a part weakness which can be addressed in advance of production. Given the various benefits, regulators encourage the use of FEA as a development and design optimization tool. Your product will more likely pass any tests and go through the FDA’s stringent design controls processes.
In sum, planning and proactivity are key in medical device engineering and conducting an FEA is one way to ensure that the project proceeds correctly from the very beginning.
FEA improves the efficiency of the medical device design process, and, furthermore, it is the ideal tool to better understand product requirements, thus enabling the design and development of innovative medical devices.
At Creanova, FEA is included in our medical device development process. It allows us to rapidly develop new solutions that push materials and geometries to their full potential, improve the user experience, make the patient safer and save costs.
If you need expert advice on the development of your innovative medical device, Creanova supports Med-Tech companies throughout the entire medical device development process, with the aim of bringing your medical device to optimised performance and efficiency.
We can support your work from the earlier stages of requirements definition and feasibility study, through industrial design, medical device engineering and FEA, to prototyping and contract manufacturing (ISO 13485 certified).
So, contact us and find out how Creanova can help you.