Portable Innovation, Mobility & Balance for Neurological Care
For a better quality of life
Portable Innovation, Mobility & Balance for Neurological Care
For a better quality of life
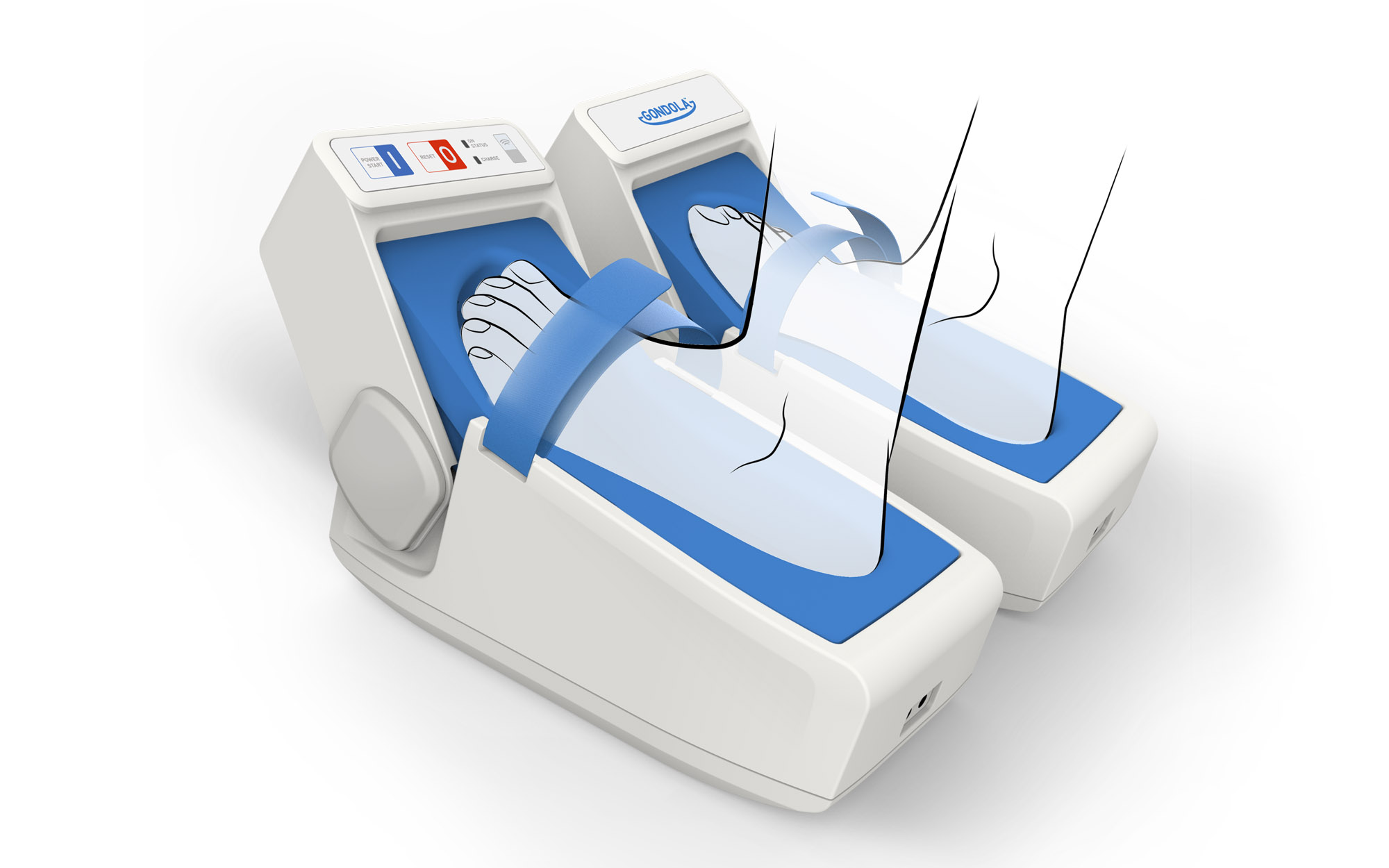
AMPS Treatment – Automated Mechanical Peripheral Stimulation Device
Gondola Medical Technologies is a Swiss start-up which stands out for its unique non-intrusive technology named AMPS (Automated Mechanical Peripheral Stimulation), treating walking and balance impairment due to neurological diseases, such as Parkinson’s and stroke.
The product consists of two devices, one for each foot, and in two versions, one for B2B market (hospitals, clinics) and one for B2C market (homecare).
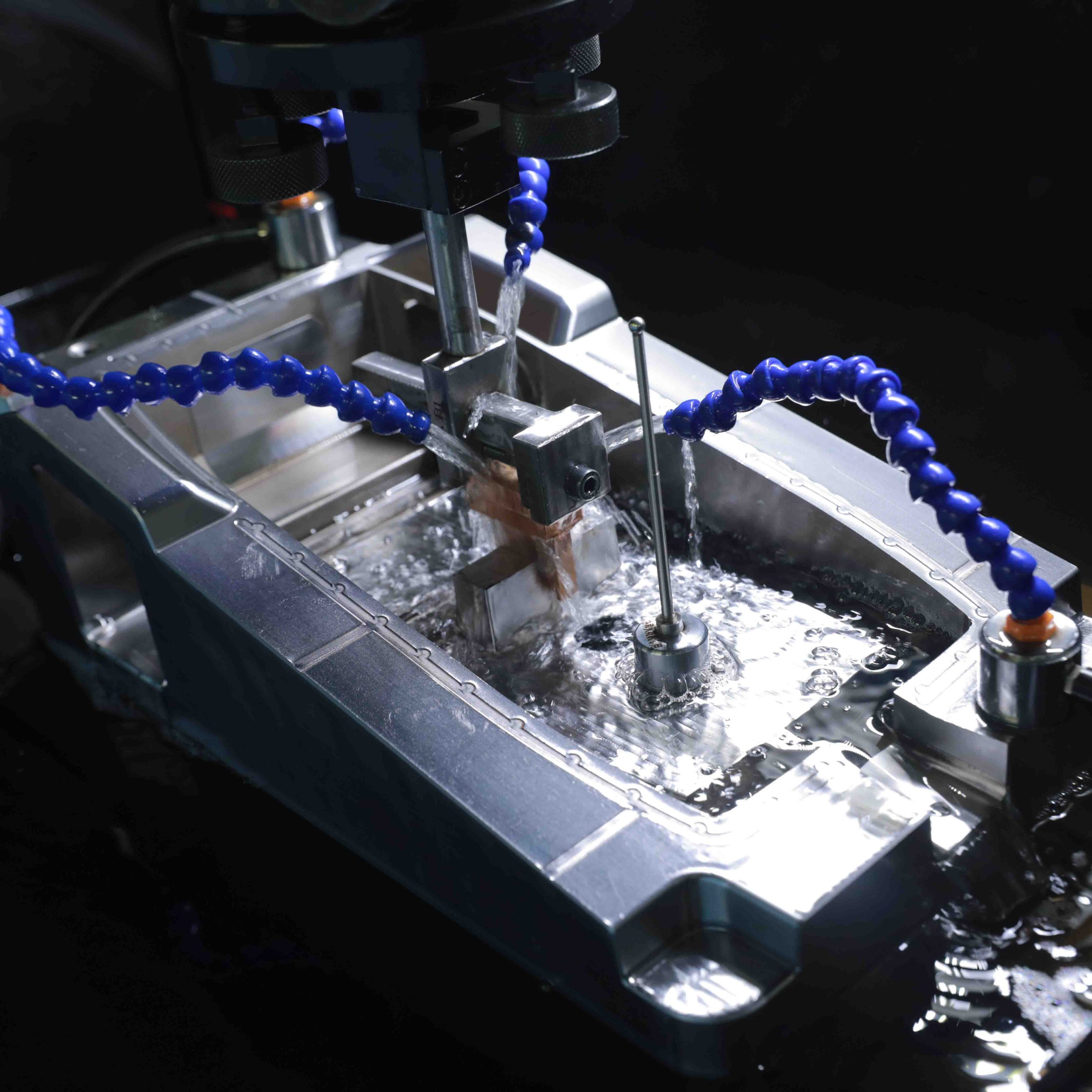
Creanova supported Gondola with R&D activities, design and engineering, with a special focus on usability and also took care of the contract manufacturing of the devices at its Italian ISO13485 certified assembly site, completing its full turn-key service from design to contract manufacturing.

Layout Analysis and R&D behind the Medical Device Design
The design and layout analysis phase required R&D activities to develop the innovative medical solution and respond to the following challenges:
- suitability for a wide range of percentiles: from the first pediatric range to feet size 47 (EU) and for patients with joint deformation
- design characterized by medical and professional looking
- the best usability for both B2B medical device and Homecare one
Our designers and engineers’ team implemented new functionalities to allow the easy adjustment of the medical device for each patient and reach the best accuracy.
On the other hand, the best usability was achieved through the analysis of:
- Patient user experience during the set-up and the treatment
- Healthcare professional experience through the following features: Speed / easiness of set-up, Management, Cleanliness
Regarding the engineering of the B2B healthcare device, the user journey was shaped to make it as easy-to-use as possible and leveraging icons and colour to make the operations intuitive and the usability effective.
On patient side, great attention was given to the comfort and softness of the footprint. The medical device has been lightened and a base was added to make it self-supporting during the therapy and finally relieve the patient.
On the other side, the Homecare device is characterized by clean and compact design and the procedure is extremely simplified so that the patient just closes the band and presses the Start button.
The layout and design phase was validated through a POC (proof o concept) to practically assess the ergonomics, volume mechanism and functioning.
Engineering: Small, Fast and with a Look to the Next Steps
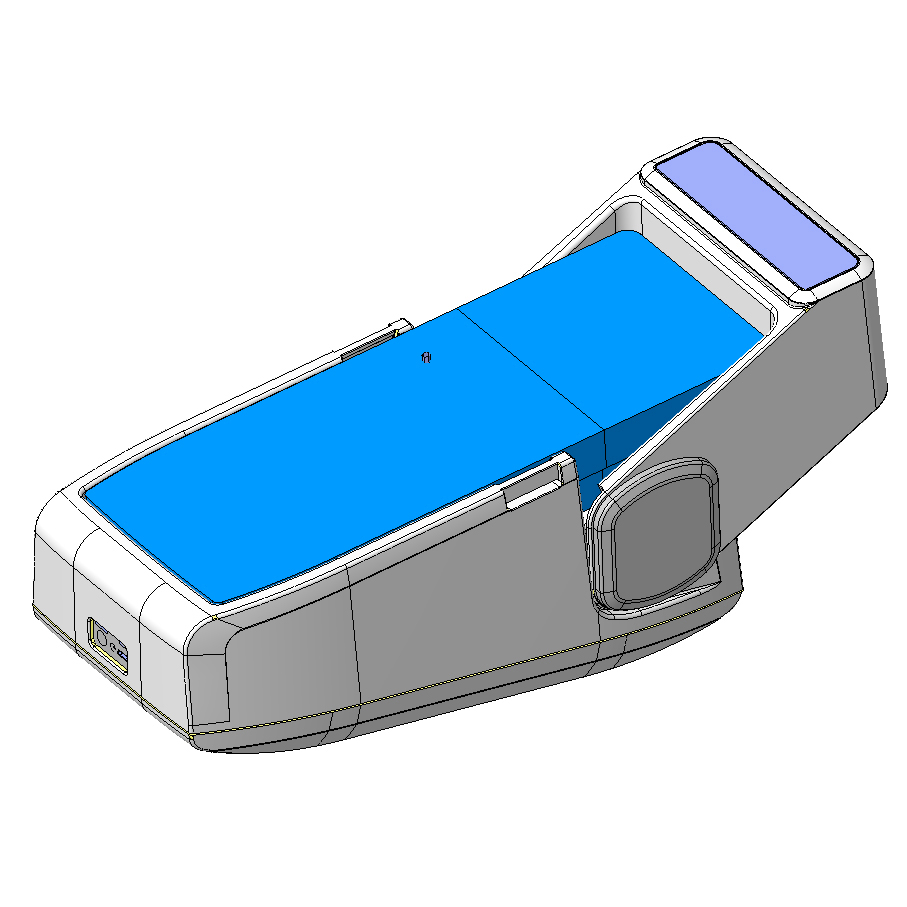
The engineering phase of the wearable medical device revolved around four main goals:
- 3D model of all housing parts and definition of mechanisms for the adjustment to each foot
- Size reduction to make the medical device portable and small enough to fit the hand luggage
- First products release by 4 months from the project starting, in order to have a prototype to submit to the Notify Body
- Identify the best production technology to reduce the initial investment
- Compliance with both European and FDA regulations
Our engineering team has made possible the easy device adjustment, without the need of any tool. For cost optimization, the two elements of each device were designed using the same shells. In addition, the silicone injection molding were chosen as the production technology to reduce initial investment.
Validation Prototype for Medical Device Certification
Functional prototypes were used to verify with the Client the usability and functionality and to submit the medical devices to the Notify Body.
These validation prototypes were subjected to several tests, aimed to assigning the medical certification to the device:
- Drop test
- IP test (Homecare device)
- Biocompatibility Test
- Electromagnetic compatibility
- Flammability tests

Complete Contract Manufacturing service
Our production specialists managed the tooling, production and assembly of this innovative wearable device, providing the following services:
- Plastic parts: plastic injection molding
- Metal parts: bending, laser cut, painting
- Laser printing of logo
- Complete assembly (electronics, mechanics and housing parts) in Creanova’s Italian assembly site, certified with ISO 13485