RESCUE SAM 4.0 - Homecare Defibrillator


One more time, Progetti Medical relied in Creanova’s experience for the development of a homecare device, the new addition to its defibrillators’ line. Progetti Medical is a leader in defibrillation systems and Rescue Sam 4.0 is the response to the increasing need of homecare medical devices.
In this project our full solution (AED design, AED engineering, prototyping, defibrillator contract manufacturing and assembly) was provided to market a compact, portable and with high IP level medical device.

Designing for homecare defibrillation
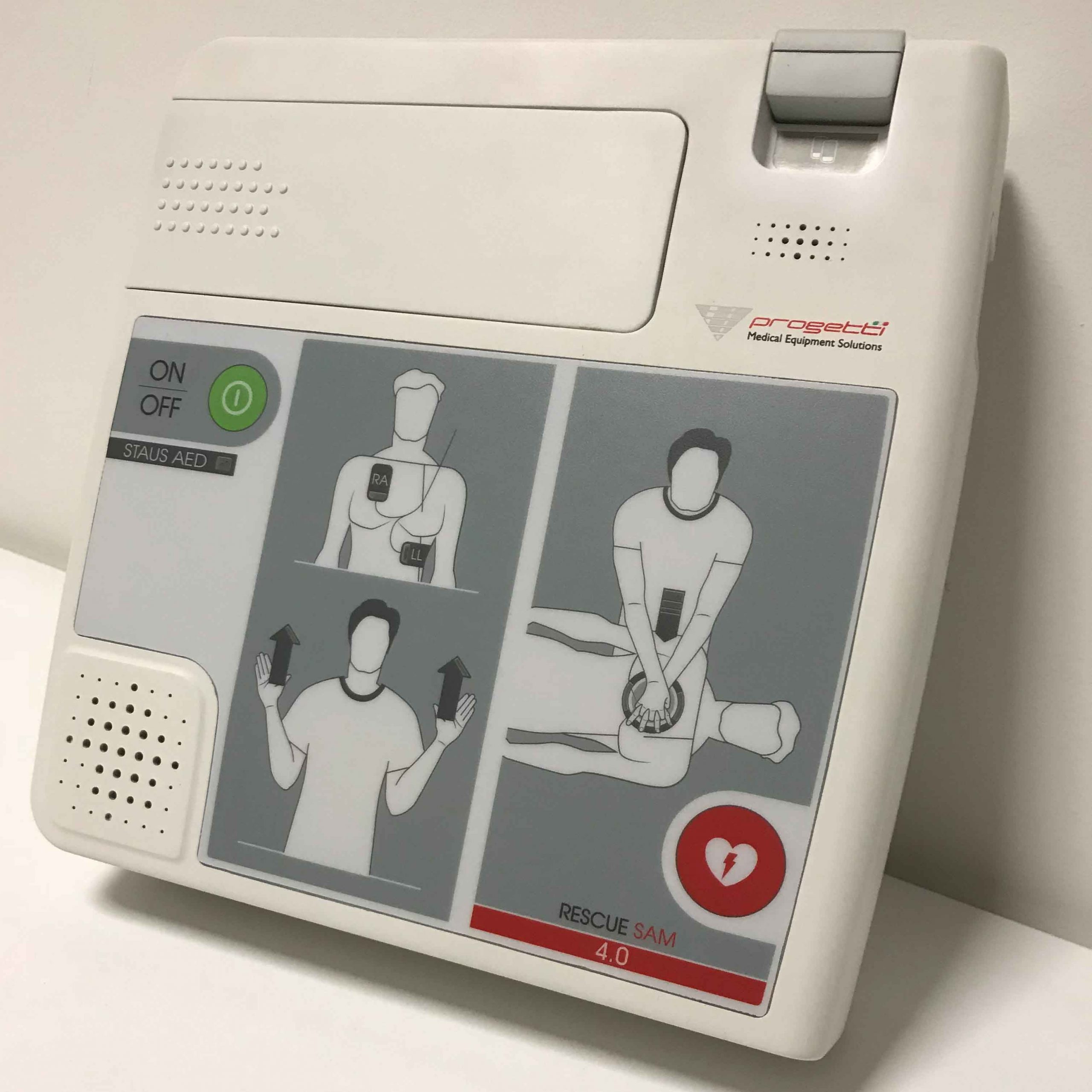
Born for the homecare, the concept of Rescue Sam 4.0 revolves around clean lines, shades of white and a sober graphic style, characteristics that make it fully integrated in the home environment while making it starkly different from the professional defibrillators.
This AED is available in two versions: with mylar and with monitor, which safely drive the user through life-saving resuscitation. The user-friendly graphics placed on the mylar consists in an intuitive visual aid throughout the procedures, which leverages leds to notify the move to the next step. With an eye towards usability, this defibrillator was designed to be handheld and hung on the wall.

Engineering vs. dust and water
Creanova’s engineering team ensured the protection against dust and jets of water according to IP55, paying a special attention to the sealing system and to the main shells modelling.
Looking towards the production and aspiring to cost optimization, our product engineers had applied a technical expedient to use the same top cover for both the versions (mylar/ monitor). Intended for unprofessional users, this medical device is equipped with fast release hook-up system for the battery.
CNC milled prototypes are provided to test the assembly procedure and to present Rescue Sam 4.0 in the medical tradefairs.
"Creanova is an innovative company able to catch what you need and create together products you dreamt."
Ivan Mangone
General Manager of Progetti Medical
Contract Manufacturing: injection molding and FIPFG
Mold making and manufacturing of Rescue Sam 4.0 was made challenging by the high number of internal elements. In the manufacturing phase, the water and dust protection was allowed through the formed in place foam gasket (FIPFG) along the medical device’s perimeter. To achieve the same goal, in our Serbian assembly site (certified with ISO 13485) the battery has undergone ultrasonic welding.