Your gateway to advanced packaging solutions for medical devices
In the design of medical devices, attention to packaging is not a negligible detail, but an essential component. An effective packaging solution does not just protect the product but also safeguards the patient’s health and facilitates the work of healthcare personnel. At Creanova, we design packaging that meets the stringent needs of a highly regulated sector and that integrates perfectly with the usability of the device itself. Let’s explore together the four pillars on which packaging design is based that really make the difference.
Patient and operator safety
The primary function of packaging is to ensure that the medical device reaches the point of use in optimal conditions of integrity and functionality and, when required, sterility. This means designing packaging that guarantees correct functioning. The secondary, but more important, function is the safety of the patient and operator to ensure effective and non-harmful treatments.
It all starts with the choice of materials, which must offer different levels of protection:
- mechanical, shock and vibration
- physical, temperature and humidity
- chemical, bacteria
To ensure the functioning of these materials, it is essential to integrate tamper-evident closure systems.
Sterilization is the most challenging process from a design point of view, but the one that guarantees the best integrity of the product.

The Creanova production plant is equipped with a clean room, making us the ideal partner for the creation and packaging of your sterile product.
The role of performance tests is fundamental to verify the effectiveness of the protective seal of the packaging and guarantee safety.
Regulatory Compliance: Designing for Global Standards
Any medical device packaging must meet a complex set of international regulations that ensure: safety, labeling, sterilization and process validation. Certifications such as ISO 11607 (for sterile packaging), the European MDR Regulation and the FDA in the United States require a rigorous and documented approach.
This involves:
- process validation through protocols
- detailed technical documentation
- compliant labeling and UDI identification for traceability
At Creanova, our integrated design approach includes complete management of documentation from the early stages of the project, reducing the risk of delays in obtaining regulatory approvals.
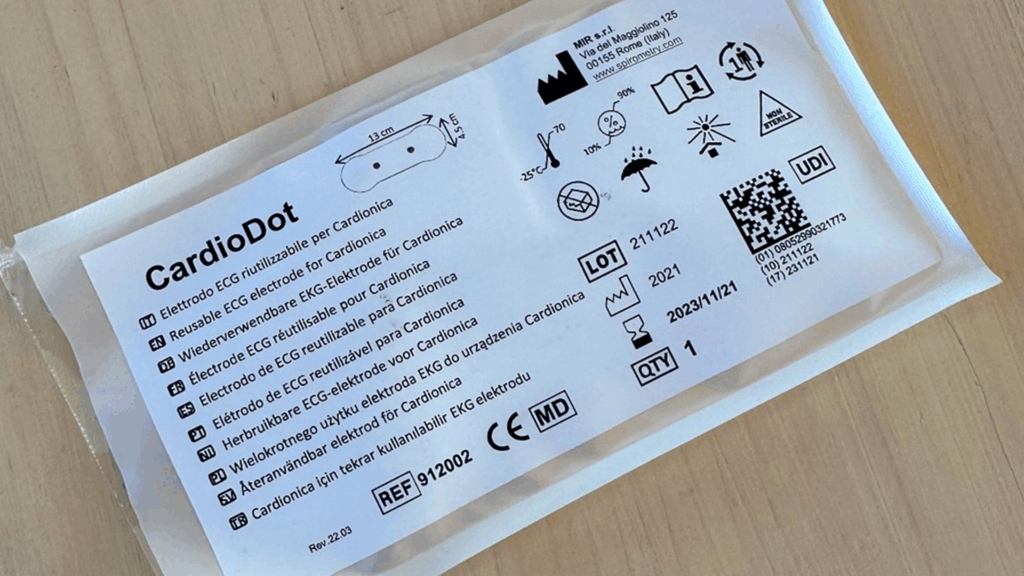
Usability and reduction of human error: packaging as an interface
In the medical sector, packaging is not a neutral element, on the contrary, it is the first physical interface between the user and the product. For this reason, it must intuitively guide the user, doctor, nurse or patient, towards correct and safe use. You can help reduce the risk of human error through effective design made up of:
- clear and visible instructions
- easy opening systems (avoiding forcing, contamination or errors)
- ergonomics of use
- easily recognizable color codes and pictograms
We think about every visual detail and to optimize the interaction, in line with the principles of our user-centered design, not for mere decoration in itself.

Packaging design: when form, regulation and safety meet
Packaging design for medical devices is a transversal discipline, it requires a balance between:
- design
- ergonomics
- regulation
- visual communication.
It is not just about “dressing” a product, but creating a system that communicates clearly, protects effectively considering the entire life cycle of the device: from production to storage, up to use.
To design excellent packaging, our design team must:
- reflect the identity and quality of the brand
- make the content and method of use immediately understandable.
- facilitate the workflow of the healthcare professional.
- guarantee product protection, sterility, safety
- comply with standards and traceability regulations.
For this reason, at Creanova, we adopt a holistic approach right from the preliminary stages, ensuring that every aesthetic or functional choice is consistent with all needs.

In such a regulated and complex context, medical device packaging cannot be an afterthought.
It must be an integral part of the project from the initial stages, with a vision capable of combining regulations, safety, usability, sustainability through an attractive aesthetic.
With our experience in medical device design, we develop customized packaging solutions that reflect the product’s identity and improve user interaction, contributing to a safe and intuitive user experience, from the warehouse to the operating room.
Contact us to explore how we can support your medical device packaging design

