Injection-molded plastic components have long been the choice of medical device contract manufacturers for projects requiring tight tolerances, complex product geometries, and lightweight versatility. This level of confidence is credited to things such as trusted designs and collaborations with an experienced medical device contract manufacturer, but one key contributor to production consistency that is often overlooked is Moldflow analysis.
Ideally, when developing devices that will be made of plastics and thus require molding of parts, an experienced contract manufacturer should be included in the project concept and design phase as well as throughout production. Their expertise in plastics, part design, and engineering is invaluable in avoiding delays, added costs, and potential dangers of designs that aren’t well suited for the injection molding process.
What Is Mold Flow Analysis?
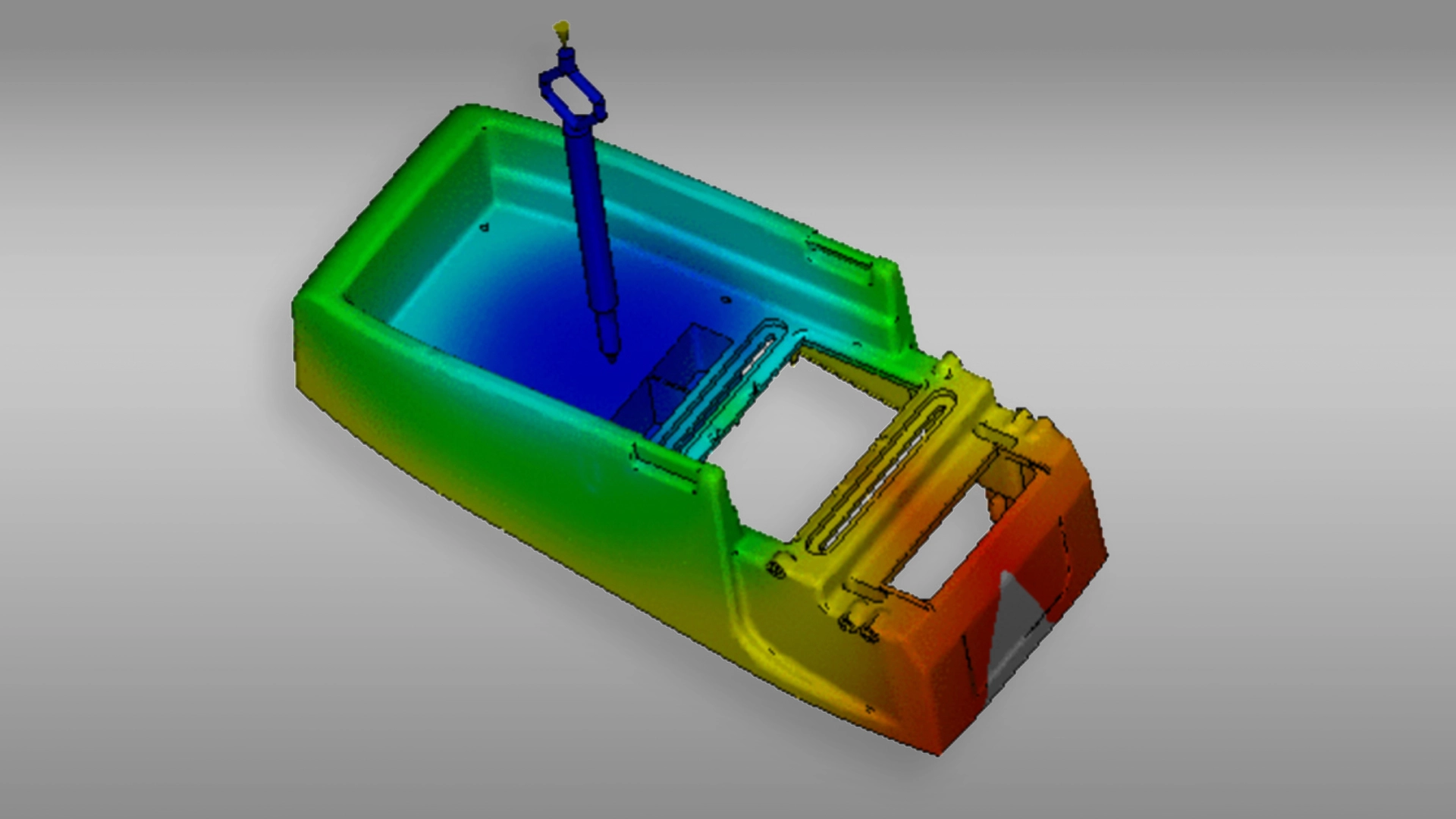
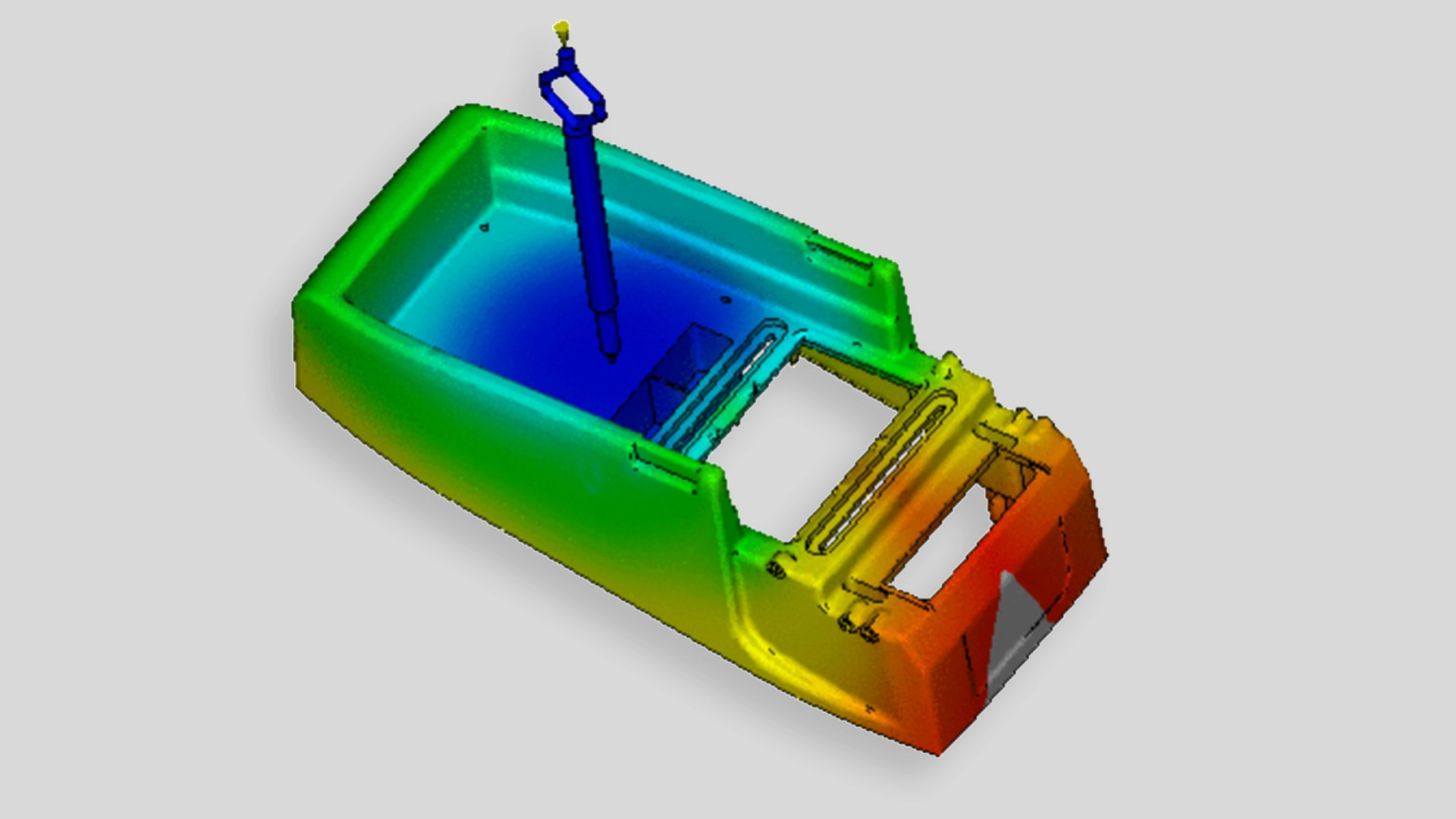
Mold Flow Analysis is a special simulation to verify parts’ behavior during and after the molding process, allowing to reinforce mold design and make the very best quality products possible.
A mould design is based on the desired plastic product’s 3D drawings. Nevertheless, there are always uncertainties (although sometimes minor, they are no less important) concerning the final functioning of the mould, as every product and every mould are different. Many variables influence the final shape and possibly also the functioning of the product. A mould flow analysis literally shows how the (liquid) plastic will flow into the mould and how the plastic will then cool and ‘harden’. In this way, the injection moulding process can be fine-tuned even before it has even begun.
Which Are The Benefits Of Mold Flow Analysis For Medical Devices?
Mould flow analysis predicts a lot about the process of medical device plastic injection molding and perfects the medical injection moulding process before it has begun.
Moldflow analysis is a valuable tool allowing the ability to scrutinize every aspect of new or existing injection molding project designs. Being able to more definitively answer project-specific questions about fill/flow progressions, gating, cooling, shrinkage and warpage:
- Prevents costly design defects from arising that compromise performance in critical-use medical situations and put brands at risk of legal action
- Ensures faster time to market by revealing design errors before tooling is built (fixing design errors after tooling is costly in terms of time and money)
- Maintains quality standards through consistently tight tolerances, repeatable tooling, and prediction/eradication of flow lines, weld lines, warping, shrink marks, and other flaws
An experienced medical device contract manufacturer that provides Moldflow analysis into their DfM and overall molding processes is your key to reliable medical components and devices that help ensure faster time to market, greater competitive advantage, and higher profitability.
Which Are The Benefits Of Creanova?
Through the mould flow analysis, Creanova helps to make molds of the very best quality, maximize product performance and appearance, improve manufacturability and choose the most suitable materials.
Our engineers transform the findings of the analysis in changes/improvements to be applied to molds, so as to possess the specified product.
We are focused on the production of highly precise plastic medical devices and we guide our clients through all phases of production: medical injection molding, mold making, Parts production, PCB production and programming, Quality Control, Assembly, Cleanroom production & assembly, Testing and validation of assembled device, Product traceability (ISO 13485), Labelling & Packaging, Storage & Logistics, Medical device Tooling/ Mold Making.
We also provide industrial design, engineering, prototyping and R&D support which make Creanova a full-service medical device product development and contract manufacturing company.